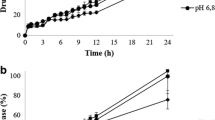
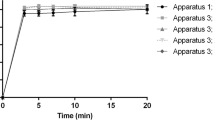
Experimental data on the comparative dissolution kinetics of two ibuprofen preparations from different manufacturers are presented. The study was performed in three buffer solutions at pH 1.2, 4.5, and 6.8 according to requirements of the Scientific Center for Expert Evaluation of Medicinal Products of the Russian Federation. A fourth dissolution medium was buffer solution at pH 7.2, which is included in a draft regulation. The test used a Erweka DT-720 rotating-basket device.




Similar content being viewed by others
References
O. Yu. Baula, Current Regulatory Requirements for Research and Registration of Generic Drugs [in Russian], Farmsodruzhestvo, Moscow (2007).
Directive 2004 / 27 / EC of the European Parliament and of the Council, Art. 10.1 (2004).
A. I. Tentsova and I. S. Azhgikhin, Dosage Form and Therapeutic Efficacy of Drugs. (Introduction to Biopharmacy) [in Russian], Meditsina, Moscow (1974), pp. 57 – 58.
V. G. Kukes and V. P. Fisenko (eds.), Bioequivalence Assessment of Drugs. Methodical Instructions [in Russian], Ministry of Health and Social Development (MHSD) of the RF, Moscow (2008).
A. N. Mironov (ed.), Guide for Drug Review [in Russian], Vol. 3, Poligraf-plyus, Moscow (2014), p. 218.
V. Shah, Y. Tsong, P. Sathe, and R. Williams, Dissolution Profile Comparison Using Similarity Factor f 2, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), FDA (2004).
Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms (1997).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 3, pp. 50 – 53, March, 2018.
Rights and permissions
About this article
Cite this article
Prozorova, N.A., Vdovina, G.P. & Bobrov, A.V. Comparative Dissolution Kinetics of Ibuprofen 200-Mg Capsules (Medisorb Co., Russia) and Ibuprofen 200-Mg Coated Tablets (Biosynthesis Co., Russia). Pharm Chem J 52, 245–247 (2018). https://doi.org/10.1007/s11094-018-1800-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1800-y