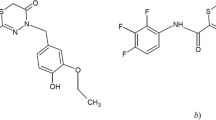
A new GC-MS method for determining an antiviral agent (camphecin) in blood plasma was developed. Sample preparation consisted of precipitating proteins using MeOH. The method was validated for selectivity, calibration curve, accuracy, precision, limit of quantitation, carryover, and stability. The analytical range was 0.1 – 2 μg/mL. The method could be used for pharmacokinetic studies.






Similar content being viewed by others
References
J. Yu, D. Wang, J. Jin, et al., Antiviral Res., 127, 68 – 78 (2016).
O. I. Kiselev, Pregnancy, Immunosuppression, Flu and Placental Expression of Endogenous Retroviruses [in Russian], Rostok, St. Petersburg (2014).
E. De Clercq, Nat. Rev. Drug Discovery, 5, 1015 – 1025 (2006).
C. Scholtissek, G. Quack, H. D. Klenk, et al., Antiviral Res., 37, 83 – 95 (1998).
A. S. Sokolova, O. I. Yarovaya, N. F. Salakhutdinov, et al., Bioorg. Med. Chem., 22(7), 2141 – 2148 (2014).
A. S. Sokolova, O. I. Yarovaya, N. F. Salakhutdinov, et al., Bioorg. Med. Chem., 21(21), 6690 – 6698 (2013).
A. S. Sokolova, O. I. Yarovaya, N. F. Salakhutdinov, et al., Eur. J. Med. Chem., 105, 263 – 273 (2015).
V. V. Zarubaev, T. S. Tretiak, N. F. Salakhutdinov, et al., Antiviral Res., 120, 126 – 133 (2015).
S. N. Bykovskii, I. A. Vasilenko, et al., Guide to Instrumental Methods for Drug Research and Development and Quality Control [in Russian], Pero, Moscow (2014).
Guidance for Industry: Bioanalytical Method Validation, U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evolution and Research (CDER), U. S. Government Printing Office, Washington, DC (2001).
Guideline on Validation of Bioanalytical Methods, European Medicines Agency, Committee for Medicinal Products for Human Use, London (2009).
Acknowledgments
The work was sponsored by RSF Grant 15-13-17.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 51, No. 12, pp. 30 – 33, December, 2017.
Rights and permissions
About this article
Cite this article
Sokolova, A.S., Yarovaya, O.I., Nefedov, A.A. et al. Development and Validation of a Method for Determination of a New Effective Inhibitor of Influenza Virus H1N1 in Blood Plasma. Pharm Chem J 51, 1102–1105 (2018). https://doi.org/10.1007/s11094-018-1748-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1748-y