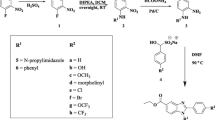
Emergence of various forms of resistant strains of Mycobacterium tuberculosis led to the exploration of drugs with novel mechanism of action. Recently econazole, an azole based antitubercular agent, attracted major attention for targeting mycobacterial cytochrome P450. In the present study, we designed novel 1,2,4-triazole derivatives based on econazole moiety and evaluated them for in vitro antitubercular activity against M. tuberculosis H37Rv and multi-drug resistant (MDR) strains of Mycobacterium.




Similar content being viewed by others
References
K. Manchester. Med. History, 28, 162 – 173 (1984).
Global Tuberculosis Report: WHO Global TB Database, Annex. 4 (2012), pp. 139 – 251.
C. Lienhardt, M. Raviglione, M. Spigelman, et al. J. Infect. Dis., 205, 241 – 249 (2012).
S. T. Cole, R. Brosch, J. Parkhill, et al., Nature, 393, 537 – 544 (1998).
K. J. McLean, Adrian J. Dunford, et al. Arch. Biochem. Biophys., 464, 228 – 240 (2007).
J. K. Capyk, R. Kalscheuer, G. R. Stewart, et al. J. Biol. Chem., 284, 35534 – 35542 (2009).
K. J. McLean, P. Lafite, C. levy, et al., J. Biol. Chem., 284, 35524 – 35533 (2009).
K. J. McLean, P. Carroll, D. Geraint Lewis, et al., J Biol Chem., 283, 33406 (2008).
H. E. Seward, A. Roujeinikova, K. J. McLean, et al., J. Biol. Chem., 281, 39437 – 39443 (2006).
P. Kale and L. B. Johnson. Drugs Today, 41, 91–105 (2005).
Z. Ahmad, S. Sharma, G. K Khuller., FEMS Microbiol. Lett., 261, 181–186 (2006).
Z. Ahmad, S. Sharma, G. K. Khuller, FEMS Microbiol. Lett., 251, 19–22 (2005).
A. Kakefuda, T. Suzuki, T. Tobe, et al. Bioorg. Med. Chem., 10, 1905 – 1912 (2002).
Q. Zhang, Y. Peng, Xin I. Wang, et al., J. Med. Chem., 50, 749 – 754 (2007).
T. J. Cheese, M. Holm, F. Lehmann, et al., J. Med. Chem., 52, 4200 – 4209 (2009).
A. R. Bhat, G. V. Bhat, and G. G. Shenoy, J. Pharm. Pharmacol., 53, 267 – 272 (2001).
M. R. Shiradkar, K. K. Murahari, H. Reddy Gangadasu, et al., Bioorg. Med. Chem., 15, 3997 – 4008 (2007).
N. B. Patel, I. H. Khan, and S. D. Rajani, Eur. J. Med. Chem., 45, 4293 – 4299 (2010).
R. J. Ganesh, M. U. Shaikh, R. Prabhakar Kale, et al., Eur. J. Med. Chem., 44, 2930 – 2935 (2009).
H. K. Foks, M. Janowiec, Z. Zwolska, and E. A. Kopeć, Phosphorus, Sulfur Silicon Relat. Elem., 180, 537 – 543 (2005).
C. Ainsworth. J. Am. Chem. Soc., 78, 4475–4478 (1956).
Y. Li, J. Liu, H. Zhang, et al., Bioorg. Med. Chem. Lett., 16, 2278 – 2282 (2006).
D. A. Charistos, G. V. Vagenas, L. C. Tzavellas, et al., J. Heterocycl. Chem., 31, 1593–1598 (1994).
H. K. Foks and B. Milczarska. Phosphorus, Sulfur Silicon Relat. Elem., 180, 197 – 204 (2005).
R. Smičius, Milda M. Burbuliene, V. Jakubkiene, et al., J. Heterocycl. Chem., 44, 279 – 284 (2007).
Jack R. Reid and Ned D. Heindel, J. Heterocycl. Chem., 13, 925 – 926 (1976).
N. F. Eweiss, A. A. Bahajaj, and E. A. Elsherbini, J. Heterocycl. Chem., 23, 1451 – 1458 (1986).
A. Rahman Farghaly, E. D. Clercq, and H. E. Kashef, Arkivoc, 10, 137 – 151 (2006).
R. J. Wallace, Jr, D. R. Nash, L. C. Steele, and V. Steingrube. J. Clin. Microbiol., 24, 976 – 981 (1986).
Lisa A. Collins and Scott G. Franzblau, Antimicrob. Agents Chemother., 41, 1004 – 1009 (1997).
F. Lombardo, M. Y. Shalaeva, K. A. Tupper, et al., J. Med. Chem., 43, 2922 – 2928 (2000).
D. Lowes, A. Pradhan, L. V. Iyer, et al., J. Med. Chem., 55, 6087 – 6093 (2012).
M. C. Alley, D. A. Scudiere, A. Monks, et al., Cancer Res., 48, 589 – 601 (1988).
D. A. Scudiere, R. H. Shoemaker, K. D. Paul, et al., Cancer Res., 48, 4827 – 4833 (1988).
F. Ardito, B. Posteraro, M. Sanguinetti, et al., J. Clin. Microbiol., 39, 4440 – 4444 (2001).
A. Kruuner, M. D. Yates, and F. A. Drobniewski. J. Clin. Microbiol., 44, 811 – 818 (2006).
BACTEC MGIT 960 Kit Protocol, BD Biosciences (2012).
A. P. Li. Drug Discovery Today, 6, 357 – 366 (2001).
P. Baranczewski, A. Stañczak, K. Sundberg, et al., Pharmacol. Rep., 58, 453–472 (2006).
Acknowledgements
The authors are grateful to the Indian Council of Medical Research (New Delhi) for funding the project (58/15/2006-BMS). They are also thankful to the Manipal University for providing facilities during the project work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ganesh Kumar, T.N.V., Gautham Shenoy, G., Kar, S.S. et al. Design, Synthesis and Evaluation of Antitubercular Activity of Novel 1,2,4-Triazoles Against MDR Strain of Mycobacterium tuberculosis. Pharm Chem J 51, 907–917 (2018). https://doi.org/10.1007/s11094-018-1714-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1714-8