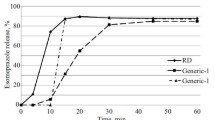
A comparative dissolution kinetics test was used to study the release (dissolution kinetics) of an original and 10 generic formulations of omeprazole from different manufacturers in a medium simulating the moderately acidic conditions in the stomach typical of the state of medication-induced suppression of acidity; tests were also performed in a model of pathological duodenogastric reflux. HPLC was used to measure omeprazole concentrations in aliquots collected at 4, 10, 15, 20, 30, 45, and 60 min in solution with pH 7.0 ± 0.05 after 2 h of exposure at pH 1.2 ± 0.05 or 4.0 ± 0.05. The duration of action of pathological duodenogastric reflux on the therapeutic formulations of omeprazole was 4 min. Not all the study formulations could be completely recognized as equivalents to the original formulation in the in vitro test conditions.


Similar content being viewed by others
References
B. K. Romanov, N. D. Bunyatyan, Yu. V. Olefir, et al., Vedom. Nauch. Tsentra Ékspert. Sredstv Med. Primenen., No. 2, 3 – 8 (2015).
D. P. Romodanovskii, D. V. Goryachev, Yu. V. Olefir, and N. D. Bunyatyan, Khim.-farm. Zh., 50(12), 42 – 45 (2016); Pharm. Chem. J., 50(12), 814 – 816 (2016).
A. V. Sokolov, Yu. B. Belousov, S. K. Zyryanov, et al., Farmakokin. Farmakodin., No. 1, 43 – 49 (2012).
D. Yu. Grebenkin, Ya. M. Stanishevskii, and I. E. Shokhin, Éffekt. Bezopasn. Lek. Sredstv, 14(1), 166 – 171 (2016).
https: //www.accessdata.fda.gov/scripts/cder/dissolution/dsp Search Results.cfm (accessed on April 26, 2017).
A. El-Sayed, N. A. Boraie, F. A. Ismail, et al., Eastern Mediter. Nealth J., 13(6), 1427 – 1434 (2007).
V. N. Satsukevich, D. V. Satsukevich, Risk Factors for Acute Complications of Gastroduodenal Ulcers [in Russian], Libereya, Moscow (1999).
K. H. Fuchs, T. R. DeMeester, R. A. Hinder, et al., Ann. Surg, 213(1), 13 – 20 (1991).
N. J. Bell, Digestion, 51(1), 59 – 67 (1993).
Guidelines for Expert Assessment of Drugs [in Russian], Grif i K, Moscow (2013), Vol. 1.
I. E. Shokhin, G. V. Ramenskaya, G. V. Vasilenko, et al., Methodological Guidelines for Drug Developers and Manufacturers for Studies of the Comparative Dissolution Kinetics of Generic Formulations, Moscow (2010).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 51, No. 9, pp. 55 – 59, September, 2017.
Rights and permissions
About this article
Cite this article
Vasilenko, G.F., Krasnykh, L.M., Serebrova, S.Y. et al. A Comparative Dissolution Kinetics Test for Omeprazole-Containing Medicines, Reproducing Secretory and Motor-Evacuatory Impairments the Stomach of Patients with Acid-Dependent Diseases. Pharm Chem J 51, 824–828 (2017). https://doi.org/10.1007/s11094-017-1700-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-017-1700-6