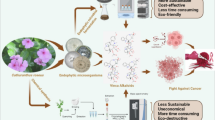
The alkaloid from the yellow waterlily nuflein, whose preparation is described here, has cytotoxic activity against HeLa cervical cancer tumor cells. Suppression of the viability of normal fibroblasts requires nuflein concentrations 67 times greater than suppression of the viability of the same number of HeLa tumor cells. Cytotoxic activity is mediated by induction of the mitochondrial apoptosis pathway, as nuflein induces opening of mitochondrial pores and release of cytochrome c, which activates caspases. Nuflein also inhibits cellular energy generation due to uncoupling of oxidative phosphorylation in mitochondria and inhibition of mitochondrial respiration. The cytotoxic action of nuflein has been suggested to result from the properties of the sulfur atom in the thioether bond in the pharmacophore molecule. Utilization of the chemical properties of nuflein, allowing its steric structure to be “tuned,” may lead to the creation of a whole series of probes with different activities directed at modifying or inhibiting biologically important thiols. The greater selectivity of the antiproliferative activity of nuflein in relation to tumor cells as compared with the known antitumor substance cisplatin points to a specific antitumor nature for the activity of nuflein.





Similar content being viewed by others
References
D. A. Murav′eva, I. A. Samylina, and G. P. Yakovlev, Farmacognosy [in Russian], Bukinist, Moscow (2002).
M. E. Perel′son, T. N. Il′inskaya, and O. N. Tolkachev, Khimiya Prirod. Soedin., 6, 768 – 770 (1975).
Russian Federation Patent RF 2292218 (2005).
Russian Federation Patent RF 549965 (1975).
A. Korotkov, N. Li, S. W, et al., Angew. Chem. Int. Ed. Engl., 54(36), 10,604 – 10,607 (2015).
A. V. Semeikin, T. A. Fedotcheva, I. S. Levina, et al., Khim.-Farm. Zh., 48(6), 9 – 13 (2014); Pharm. Chem. J., 48(6), 363 – 367 (2014).
T. A. Fedotcheva, N. L. Shimanovskii, A. G. Kruglov, et al., Biol. Membrany, 28(6), 1 – 8 (2011).
T. A. Fedotcheva, E. V. Odintsova, V. V. Banin, et al., Vestnik RONTs im. N. N. Blokhina RAMN , 4, 34 – 38 (2011).
F. Ciscato, M. Sciacovelli, G. Villano, et al., Oncotarget, 5(9), 2418 – 2427 (2014).
Y. Xu, H. B. Ma, Y. L. Fang, et al., Cell Signal., 31, 112 – 123 (2017); doi: https://doi.org/10.1016/j.cellsig.2017.01.004.
H. Matsuda, K. Yoshida, K. Miyagawa, et al., Bioorg. Med. Chem. Let., 16(6), 1567 – 1573 (2006).
S. Okamura, E. Nishiyama, T. Yamazaki, et al., Biochim. Biophys. Acta, 1850(6), 1245 – 1252 (2015); doi: https://doi.org/10.1016/j.bbagen.2015.02.012.
J. Ozer, N. Eisner, E. Ostrozhenkova, et al., Cancer Biol. Ther., 8(19), 1860 – 1868 (2009).
N. Tada, D. J. Jansen, M. P. Mower, et al., ACS Cent. Sci., 2(6), 401 – 408 (2016); doi: https://doi.org/10.1021/acscentsci.6b00113.
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 51, No. 7, pp. 34 – 39, July, 2017.
Rights and permissions
About this article
Cite this article
Fedotcheva, T.A., Sheichenko, O.P., Anufrieva, V.V. et al. Preparation of Nuflein - an Alkaloid from the Yellow Waterlily Nuphar Lutea - and its Cytotoxic Action on Cultures of Normal and Tumorous Human Cells. Pharm Chem J 51, 590–595 (2017). https://doi.org/10.1007/s11094-017-1658-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-017-1658-4