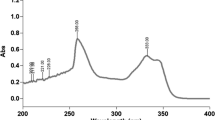
Gefitinib was determined quantitatively in a nanoformulation using the proposed HPLC method. Effective elution was achieved by using a stationary phase of HyperClone (Phenomenex®) C18 column (250 mm × 4.6 mm i.d., 5 μm, BDS 130 Å) and a mobile phase composed of acetonitrile and 40 mM ammonium formate buffer pH 2.5 (30 : 70, %v/v) at a flow rate of 1.0 mL/min. The samples were measured at 248 nm using UV detector. The column temperature was kept at 25°C; the run time was 7 min; the injection volume was 20 μL. Gefitinib was separated within 4.476 min. The correlation coefficient for the obtained calibration curve was found to be 0.998. The method was validated according to ICH Q2 (R1) guidelines for linearity, sensitivity, robustness, accuracy and precision. The LOD and LOQ were found to be 37.852 and 114.702 ng/mL, respectively.


Similar content being viewed by others
References
D. McKillop, A. D. McCormick, G. S. Miles, et al., Xenobiotica, 34(11 – 12), 983 – 1000 (2004).
A. Arora and E. M. Scholar, J. Pharmacol. Exp. Ther., 315(3), 971 – 979 (2005).
G. Zhang, X. Xie, T. Liu, et al., Cancer Chemother. Pharmacol., 72, 767 – 775 (2013).
S. V. Schaeybroeck, A. Karaiskou-McCaul, D. Kelly, et al., Clin. Cancer Res., 11(20), 7480 – 7489 (2005).
US Food and Drug Administration. Gefitinib (Iressa®), Clinical Pharmacology and Biopharmaceutics Review, (2003).
US Food and Drug Administration. Gefitinib (Iressa®), Prescribing Information (2004).
N. R. Budha, A. Frymoyer, G. S. Smelick, et al., Clin. Pharmacol. Ther., 92(2), 203 – 213 (2012).
US Food and Drug Administration, Gefitinib (Iressa®), Highlights of Prescribing Information (2015).
R. Bazak, M. Houri, S. E. Achy, et al., J. Cancer Res. Clin. Oncol., 141(5), 769 – 784, (2015).
N. S. K. Srinivas, R. Verma, G. P. Kulyadi, and L. Kumar, Int. J. Nanomed., 12, 15 – 28, (2017).
L. Z. Wang, M. Y. L. Lim, T. M. Chin, et al., J. Chromatogr. B, 879(22), 2155 – 2161, (2011).
L. Faivre, C. Gomo, O. Mir, et al., J. Chromatogr. B, 879(23), 2345 – 2350, (2011).
Y. Wang, K. Ren, Y. Yao, et al., Lat. Am. J. Pharm., 35(3), 429 – 433, (2016).
J. Dolan, A Guide to HPLC and LC-MS Buffer Selection, Ultra Inert Base-Deactivated HPLC Columns, ACE HPLC Columns (2016), 1 – 20
L. Kumar, M. S. Reddy, R. S. Managuli, and K. G. Pai, Saudi Pharm J., 23(5), 549 – 555, (2015).
T. B. Solanki, P. A. Shah, and K. G. Patel, Indian J. Pharm. Sci., 76(3), 179 – 187, (2014).
ICH Q2 (R1): ICH Harmonized Tripartite Guidelines, Validation of Analytical Procedures: Text and Methodology Q2 (R1), International Conference on Harmonization. Geneva, Switzerland, (2005).
R. K. Shirodkar, P. Shetty, L. Kumar, et al., Lat. Am. J. Pharm. 34(1), 1162 – 1166, (2015).
D. Awotwe-Otoo, C. Agarabi, P. J. Faustin, et al., J. Pharmaceut. Biomed., 62, 61 – 67, (2012).
E. A. Krylova, S. S. Kataev, Y. A. Khomov, and O. N. Dvorskaya, Pharm. Chem. J., 49(8), 49 – 54, (2015).
Acknowledgements
The authors would like to acknowledge Neon Laboratories Ltd., Mumbai, India for providing gefitinib as a gift sample. The authors would also like to thanks Manipal University, Manipal, Karnataka, India for providing research facilities to complete this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
KS, N.S., K, G.P., Verma, R. et al. Validation of HPLC Method for Quantitative Determination of Gefitinib in Polymeric Nanoformulation. Pharm Chem J 51, 159–163 (2017). https://doi.org/10.1007/s11094-017-1575-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-017-1575-6