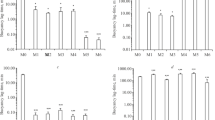
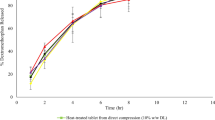
Results from technology development for manufacturing an amben prolonged-release preparation based on a Kollidon SR hydrophobic polymer matrix were presented. It was shown that dry and wet granulation could improve the processing properties of mixtures for preparing tablets and provide the required amben release kinetics.





Similar content being viewed by others
References
M. V. Leonova, Lech. Delo, No. 2, 21 – 31 (2009).
H. Wen and K. Park, Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice, JohnWiley & Sons, Hoboken, New Jersey (2010), pp. 5 – 10.
G. O. Nifontova, O. V. Dolotova, I. V. Sarantseva, et al., Khim.-farm. Zh., 50(2), 23 – 28 (2016); Pharm. Chem. J., 50(2), 90 – 95 (2016).
V. Buhler, Polyvinylpyrrolidone Excipients for Pharmaceuticals, Springer, Heidelberg (2005), pp. 217 – 218.
K. A. Khan and P. Musikabhumma, J. Pharm. Pharmacol., 33(10), 627 – 631 (1981).
F. E. Eichie, R. S. Okor, M. U. Uhumwangho, et al., Trop. J. Pharm. Res., 4(2), 483 – 487 (2005).
A. V. Son and V. A. Vainshtein, Khim.-farm. Zh., 48(1), 30 – 36 (2014); Pharm. Chem. J., 48(1), 51 – 56 (2014).
N. Tanaka, K. Imai, K. Okimoto, et al., J. Controlled Release, 112(1), 51 – 56 (2006).
Acknowledgments
The work was supported financially by the RF Ministry of Education and Science (Contract No. 02.G25.31.0001) under the auspices of RF Administration Decree No. 218 of Apr. 9, 2010, and utilized equipment of the Center for Collective Use of Unique Scientific Equipment for MPTI nanotechnology studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 50, No. 8, pp. 39 – 44, August, 2016.
Rights and permissions
About this article
Cite this article
Nifontova, G.O., Krechetov, S.P., Korostylev, E.V. et al. Development of Manufacturing Technology for Prolonged-Release Oral Amben Preparation. Pharm Chem J 50, 537–542 (2016). https://doi.org/10.1007/s11094-016-1485-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-016-1485-z