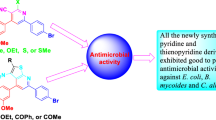
Reaction of the methyl esters of aroylpyruvic acids with mixtures of 4-(2-aminoethyl)benzenesulfonamide and an aromatic aldehyde was used to synthesize 1-[2-(4-aminosulfonylphenyl)ethyl]-5-aryl-4-aroyl-3-hydroxy- 3-pyrrolin-2-ones. The structures of these compounds were verified by IR and 1H NMR spectroscopy and mass spectrometry. Their antibacterial activity was studied.
Similar content being viewed by others
References
V. L. Gein, T. F. Odegova, K. A. Tkachenko, et al., Khim.-Farm. Zh., 47(7), 31 – 33 (2013); Pharm. Chem. J., 47(7), 371 – 373 (2013).
V. L. Gein, I. V. Kovtonogova, O. V. Bobrovskaya, et al., Zh. Obshchei Khimii, 84(2), 271 – 274 (2014).
M. D. Mashkovskii, Medicines [in Russian], RIA Novaya Volna, Moscow (2008), pp. 559 – 560.
P. B. Terent’ev, Mass Spectrometry in Organic Chemistry [in Russian], Vysshaya Shkola, Moscow (1979).
G. N. Pershin, Methods of Experimental Chemotherapy [in Russian], Meditsinskaya Literatura, Moscow (1971), pp. 100, 109 – 117.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 49, No. 9, pp. 32 – 34, September, 2015.
Rights and permissions
About this article
Cite this article
Gein, V.L., Bobrovskaya, O.V., Valiev, R.T. et al. Synthesis and Antibacterial Activity of 1-[2-(4-Aminosulfonylphenyl)Ethyl]-5-Aryl-4-Aroyl-3-Hydroxy-3-Pyrrolin-2-Ones. Pharm Chem J 49, 602–604 (2015). https://doi.org/10.1007/s11094-015-1337-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-015-1337-2