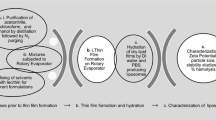
The optimum composition of a stable lyophilized liposomal formulation of tiosens is established and includes lecithin, cholesterol, and PEG-2000-DSPE in a molar ratio of 1:0.22:0.002 and sucrose solution as a cryoprotector in a 1:9 lecithin:cryoprotector weight ratio. The effects of sonication, homogenization, and filtration on the quality of the liposomal preparation were studied. The results of preliminary studies lead to a conclusion about the satisfactory storage stability of the liposome form of tiosens.
Similar content being viewed by others
References
R. R. Allison, Photodiagn. Photodyn. Ther., 6, 231 – 234 (2009).
R. R. Allison and C. H. Sibata, Photodiagn. Photodynam. Ther., 7, 61 – 75 (2010).
Z. S. Smirnova, I. G. Meerovich, and E. A. Luk’yanets, Ross. Bioterapevticheskii Zh., 1, 54 – 60 (2004).
L. G. Gatinskaya, N. A. Dmitricheva, E. V. Ignat’eva, et al., Ross. Bioterapevticheskii Zh., 1(5), 33 – 34 (2006).
I. G. Meerovich, A. A. Stratonnikov, and A. V. Ryabova, Ross. Bioterapevticheskii Zh., 3, 37 – 42 (2004).
I. G. Meerovich, Z. S. Smirnova, N. A. Obrotova, et al., Bull. Exp. Biol. Med., 139(4), 427 – 430 (2005).
I. G. Meerovich and N. A. Oborotova, Ross. Bioterapevticheskii Zh., 4, 3 – 8 (2007).
B. Mishra, B. B. Patel, and S. Tiwari, Nanomedicine: Nanotechnol., Biol., Med., 6, 9 – 24 (2010).
D. G. Gurevich, I. G. Meerovich, G. A. Meerovich, et al., Ross. Bioterapevticheskii Zh., 2, 45 – 49 (2007).
C. Chen, D. Han, C. Cai, et al., J. Controlled Release, 142, 299 – 311 (2010).
W. Abdelwahed, G. Degobert, S. Stainmesse, et al., Adv. Drug Delivery Rev., 58(15), 1688 – 1713 (2006).
Acknowledgments
The work was performed with financial support of the Moscow City Government under the auspices of the Scientific-Technical Program “Development and Practical Translation into Healthcare of New Methods and Agents for Prevention, Diagnosis, and Treatment of Oncological, Infectious, and Other Serious Diseases”.
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 45, No. 12, pp. 32 – 36, December, 2011.
Rights and permissions
About this article
Cite this article
Sanarova, E.V., Polozkova, A.P., Meerovich, I.G. et al. Influence of processing factors on the quality of the liposomal form of the new photosensitizer tiosens. Pharm Chem J 45, 741–745 (2012). https://doi.org/10.1007/s11094-012-0715-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-012-0715-2