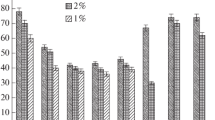
The antioxidant action of natural tyrosol derivatives, raspberry ketone, and their synthetic analogs was studied in comparison with α-tocopherol and butylhydroxytoluene. It is shown that hydroxylation of tyrosol increases the antioxidant activity 1.5 times. The introduction of one o-tert-butyl substituent increases the inhibiting action by a factor of 3.5; of two o-tert-butyl substituents, by greater than 4.0 times. The maximum inhibiting effect was observed for tert-butylhydroxytyrosol, the structural modification of which included simultaneously both hydroxylation and alkylation. The antioxidant activity of the studied substances was lower than that of α-tocopherol and butylhydroxytoluene. It was established that the induction periods of the studied antioxidants (AOs) in addition to many natural inhibitors (α-tocopherol, carotenoids, flavonoids) as functions of the concentrations of the studied AOs exhibit peaks. Thus, directed structural modifications of natural compounds yielded new effective oxidation inhibitors, the application of which will probably avoid undesired effects related to overdoses of the existing drugs.



Similar content being viewed by others
References
A. D. Barnaulov, A. Yu. Limarenko, V. A. Kurkin, et al., Farmatsiya, 43(6), 32 – 37 (1994).
A. S. Saratikov and E. A. Krasnov, Rhodiola rosea, a Valuable Medicinal Plant [in Russian], Izd. Tomsk. Univ., Tomsk (1987), p. 156.
A. Ya. Gerchikov, G. G. Garifullina, and M. M. Ishmuratova, in: Abstracts of Papers of the Vth Int. Conf. “Bioantioxidant” [in Russian], Moscow (1998), p. 33.
M. W. Lee, Y. A. Lee, H. M. Park, et al., Arch. Pharm. Res., 23(5), 455 – 458 (2000).
N. M. Storozhok, A. P. Krysin, N. V. Gureeva, et al., Khim.-farm. Zh., 36(2), 14 – 18 (2002).
A. P. Krysin, in: Abstracts of Papers of the Vth Int. Conf. “Bioantioxidant” [in Russian], Moscow (1998), pp. 50 – 51.
E. B. Burlakova, N. M. Storozhok, and N. G. Khrapova, Biofizika, 33(4), 584 – 588 (1988).
E. T. Denisov, Usp. Khim., 65(6), 547 – 563 (1996).
V. A. Roginskii, Phenolic Antioxidants: Reactivity and Effectiveness [in Russian], Nauka, Moscow (1984), p. 96.
B. Le Tutor and D. Guedon, Phytochemistry, 31(4), 1173 – 1178 (1992).
E. T. Denisov and V. V. Azatyan, Chain-Reaction Inhibition [in Russian], Chernogolovka (1997), pp. 114 – 179.
O. T. Kasaikina, V. D. Kortenska, E. M. Marinova, et al., Izv. Akad. Nauk, Ser. Khim., No. 6, 1119 – 1122 (1997).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 45, No. 12, pp. 23 – 26, December, 2011.
Rights and permissions
About this article
Cite this article
Storozhok, N.M., Gureeva, N.V., Khalitov, R.A. et al. Antioxidant activity of synthetic analogs and pure active principles of rhodiola rosea and raspberry ketone. Pharm Chem J 45, 732–735 (2012). https://doi.org/10.1007/s11094-012-0713-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-012-0713-4