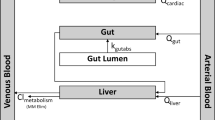
Results of QSAR research for chemical inhalation toxicity are presented. Data on the inhalation toxicity of organic compounds (403 chemicals) with respect to rats (4-h exposure) were taken from the Registry of Toxic Effects of Chemical Substances (RTECS). Among these, 28 inert chemicals with nonspecific toxicity were selected. Regression models were formed for all these compounds. The QSAR models were constructed using two approaches based on (i) experimental values of octanol—air partition coefficients and (ii) molecular physicochemical descriptors calculated by the HYBOT program. The best computer model was obtained on the basis of molecular physicochemical descriptors. A comparison of the inhalation toxicity of all examined compounds with the base nonspecific toxicity identified additional compounds with toxicity close to the base level that were not inert; a class of compounds with substantially greater toxicity (specific toxicants); and a class of compounds with substantially less toxicity due to metabolism. Satisfactory classification and regression QSAR models were obtained for 135 substances in the examined set with similar structures.
Similar content being viewed by others
References
Commission of the European Communities. Regulation (EC) No. 1907 / 2006 of the European Parliament and of the Council of 18 December 206 Concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH).
I. I. Lessigiarska, T. Netzeva, and A. P. Worth, QSAR Comb. Sci., 27(1), 41 – 48 (2008).
RTECS (Registry of Toxic Effects of Chemical Substances), Cinnicinnati, OH, NIOSH (2009); http://www.cdc.gov/niosh/rtecs/.
O. A. Raevsky, V. Yu. Grigor’ev, and S. V. Trepalin, HYBOT (Hydrogen Bond Thermodynamics) Program Package, RF Pat. No. 990,090 (1999).
O. A. Raevsky, V. A. Gerasimenko, and S. V. Trepalin, MOLDIV (Molecular DIVersity & Similarity), RF Pat. No. 990,093 (1999).
J. de Jough, H. J. M. Verhaar, and J. L. M. Hermens, Toxicol. Sci., 45(1), 26 – 32 (1998).
L. S. McCarty and D. Mackay, Environ. Sci. Technol., 27(12), 1719 – 1728 (1993).
H. J. M. Verhaar, C. J. van Leeuven, and J. L. M. Hermens, Chemosphere, 25(5), 471 – 491 (1992).
W. De Wolf, P. H. Lieder, and J. D. Walker, QSAR Comb. Sci., 23(7), 521 – 524 (2004).
O. A. Raevsky, V. Yu. Grigor’ev, E. E. Weber, and J. C. Dearden, QSAR Comb. Sci., 27(11/12), 1274 – 1281 (2008).
O. A. Raevsky, V. Yu. Grigor’ev, J. C. Dearden, and E. E. Weber, QSAR Comb. Sci., 28(1), 1 – 12 (2009).
O. A. Raevsky, A. N. Razdol’skii, V. D. Tonkopii, et al., Khim.-farm. Zh., 42(6), 22 – 27 (2008).
O. A. Raevsky and J. C. Dearden, SAR QSAR Environ. Res., 15(5,6), 433 – 448 (2004).
Acknowledgments
The work was supported financially by the ISTC (Project No. 3777).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 45, No. 3, pp. 36 – 40, March, 2011.
Rights and permissions
About this article
Cite this article
Raevsky, O.A., Modina, E.A. & Raevskaya, O.E. QSAR models of the inhalation toxicity of organic compounds. Pharm Chem J 45, 165–169 (2011). https://doi.org/10.1007/s11094-011-0585-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-011-0585-z