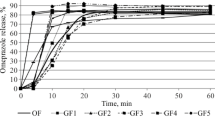
The pharmaceutical equivalence of Zantac (reference drug) and 10 domestic and foreign generics of ranitidine hydrochloride as 150-mg coated tablets has been studied using the pharmacopoeic (USP 29) dissolution test. Analyses showed insignificant differences in the excipients entering into the compositions of ranitidine generic tablets registered in Russia. It is established that Zantac and generics of two manufacturers are rapidly soluble (according to the WHO classification). Analysis of the similarity coefficients determined for the dissolution profiles measured in media with different pH values showed the biological nonequivalence of some generics and the reference drug. It is demonstrated that the in vitro dissolution test recommended by WHO can be used for determining the bioequivalence of ranitidine generics.
Similar content being viewed by others
References
B. V. Gerasimov, M. V. Zhuravleva, and A. S. Rumyantsev, Vedomosti NTs ESMP, No. 1, 37 – 40 (2007).
Annex 7. Multisource (Generic) Pharmaceutical Products: Guidelines on Registration Requirements to Establish Interchangeability. WHO Technical Report Series, No. 937 (2006).
V. L. Bagirova, L. N. Vzorova, L. K. Grakovskaya, et al., Khim.-farm. Zh., 35(4), 39 – 41 (2001).
Decree of the Russian Federation Government No. 376-r of March 29, 2007.
WHO Technical Report Series, No. 937, Annex 8 (2006).
Order of the Ministry of Health and Social Development of the Russian Federation, No. 665, September 18, 2006.
U. S. Pharmacopoeia, 29th Revision (2006).
H. Kortejarvi, M. Yliperttula, J. B. Dressman, et al., J. Pharm. Sci., 94(8), 1617 – 1625 (2005).
M. H. Gschwend, R. Guserle, A. Erenmemisoglu, et al., Arzneim. Forsch., 57(6), 315 – 319 (2007).
L. X. Ju, J. T. Wang, and A. S. Hussain, AAPS PharmSci, 4(1), 1 – 5 (2002).
V. V. Kugach and Zh. Konstantin, Vestn. Farmatsii, No. 2, 72 – 79 (2006).
J. Albers, K. Knop, and P. Kleinebudde, Pharm. Ind., 68(12), 1420 – 1428 (2006).
I. V. Voskoboinikova, S. B. Avakyan, T. A. Sokol(skaya, et al., Khim.-farm. Zh., 39(1), 22 – 28 (2005).
I. V. Voskoboinikova, S. B. Avakyan, T. A. Sokol(skaya, et al., Farmatsiya, No. 2, 35 – 37 (2005).
FDA Inactive Ingredient Database [Electronic resource], http://www.fda.gov/cder/iigfaqWEB.htm [http://www.fda.gov/Drugs/InformationOnDrugs/ucm113978.htm].
Letter of The Federal Service for Oversight of Health and Social Development No. 01I-343 / 05 of July 13, 2005 (2005).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 43, No. 11, pp. 44 – 48, November, 2009.
Rights and permissions
About this article
Cite this article
Smekhova, I.E., Moldaver, B.L. & Perova, Y.M. Equivalence of ranitidine generic tablets studied using the in vitro dissolution test. Pharm Chem J 43, 632–636 (2009). https://doi.org/10.1007/s11094-010-0368-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-010-0368-y