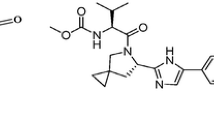
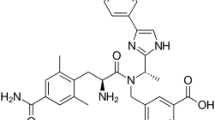
Anew analytical method using reversed-phase high performance liquid chromatography (HPLC) has been developed for analysis of four antiretroviral molecules Lamivudine, Abacavir, Zidovudine, and Efavirenz. The proposed method was validated in accordance with the ICH Q2 (R1) validation guidelines by determining its selectivity, linearity, accuracy, and precision. Based on the results, the method is an effective choice to analyze these drugs in their single and combined pharmaceutical dosage forms.
Similar content being viewed by others
References
J. Darbyshire, Drug, 45, 1 – 3 (1995).
S. M. Hammer, D. A. Katzenstein, M. D. Hughes, et al., N. Engl. J. Med., 335, 1081 – 1090 (1996).
M. H. Clair, K. N. Pennington, J. Rooney, et al., J. Acquired Immune Defic. Syndr. Hum. Retrovirol., 10, S83 – 91 (1995).
Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Available at http://www.aidsinfo.nih.gov. In: Services, DoHaH (ed.) (2005).
Monographs, in: International Pharmacopoeia, 4th ed., World Health Organization, WHO Expert Committee, Geneva (2005), p. 4.
A. S. Pereira and R. R. Tidwell, J. Chromatogr. B, 764, 327 – 347 (2001).
E. K. Kano, C. H. D. R. Serra, E. E. M. Koono, et al., Int. J. Pharmacol., 297, 73 – 79 (2005).
R. M. W. Hoetelmans, M. Profijt, P. L. Meenhorst, et al., J. Chromatogr. B, 713, 387 – 394 (1998).
D. M. Morris and K. Selinger, J. Pharm. Biomed. Anal., 12, 255 – 264 (1994).
P. H. Hsyu and T. L. Lloyd, J. Chromatogr. B, 655, 253 – 259 (1994).
A. J. Harker, G. L. Evans, A. E. Hawley, et al., J. Chromatogr. B, 657, 227 – 232 (1994).
G. Bahrami, S. Mirzaeei, A. Kiani, et al., J. Chromatogr. B, 823, 213 – 217 (2005).
B. K. Kathryn, A. W. Stephen, M. C. Richard, et al., J. Pharm. Biomed. Anal., 22, 967 – 983 (2000).
G. Aymard, M. Legrand, N. Trichereau, et al., J. Chromatogr. B, 744, 227 – 240 (2000).
A. Simon, M. D. Thiam, and L. C. Lepford, J. Chrom. A., 913, 447 – 453 (2001).
N. L. Rezk, R. R. Tidwell, and A. D. M. Kashuba, J. Chromatogr. B, 791, 137 – 147 (2003).
S. Notari, A. Bocedi, G. Ippolito, Narciso, et al., J. Chromatogr. B, 831, 258 – 266 (2006).
C. P. W. G. M. V. Wissen, R. E. Aarnoutse, and D. M. Burger, J. Chromatogr. B, 816, 121 – 129 (2005).
B. Uslu and S. A. Ozkan, Anal. Chem. Acta, 466, 175 – 185 (2002).
B. Fan and J. T. Stewart, J. Pharm. Biomed Anal., 28, 903 – 908 (2002).
J. R. Ravitch and C. G. Moseley, J. Chromatogr. B, 762, 165 – 173 (2001).
U. Yoshiko, O. Tsuyoshi, N. Masahiko, et al., Chem. Pharm. Bull., 51, 715 – 718 (2003).
T. Masaaki, Y. Masao, O. Tsuyoshi, et al., Biol. Pharm Bull., 28, 1286 – 1290 (2005).
N. L. Rezk, R. R. Tidwell, and A. D. M. Kashuba, J. Chromatogr. B, 913, 241 – 247 (2004).
D. R. Weller, R. C. Brundage, H. H. Balfour, and H. E. Vezina, J. Chromatogr. B, 848, 369 – 373 (2007).
A. Dunge, N. Sharda, B. Singh, and S. Singh, J. Pharm. Biomed Anal., 37, 1109 – 1114 (2005).
P. J. Wagh and N. Pai, Indian Drugs, 39, 654 – 657 (2002).
R. Sekar and S. Azhaguvel, J. Pharm. Biomed Anal., 39, 653 – 660 (2005).
D. Predrag, L. Aleksandra, M. Slavko, and J. S. Milena, Anal. Lett., 37, 2649 – 2667 (2004).
H. Rebiere, B. Mazel, C. Civade, and P. A. Bonnet, J. Chromatogr. B, 850, 376 – 383 (2007).
S. Anbazhagan, N. Indumathy, P. Shanmugapandiyan, and S. K. Sridhar, J. Pharm. Biomed Anal., 39, 801 – 804 (2005)
M. Sarkar, S. Khandavilli, and R. Panchagnula, J. Chromatogr. B, 830, 349 – 354 (2006).
N. Kapoor, S. Khandavilli, and R. Panchagnula, J. Pharm. Biomed Anal., 41, 761 – 765 (2006).
N. Kapoor, S. Khandavilli, and R. Panchagnula, Anal. Chim. Acta, 570, 41 – 45 (2006).
K. Keil, V. A. Frerichs, R. DiFrancesco, and G. Morse, Ther. Drug Monitor., 25, 340 – 346 (2003).
E. Dailly, F. Raffi, and P. Jolliet, J. Chromatogr. B, 813, 353 – 358 (2004).
J. M. Poirier, P. Robidou, and P. Jaillon, Ther. Drug Monitor., 27, 186 – 192 (2005).
I. Schrive and J. C. Plasse, J. Chromatogr. B, 657, 233 – 237 (1994).
F. Kamali and M. D. Rawlins, J. Chromatogr. B, 530, 474 – 479 (1990).
C. Lacroix, T. P. Hoang, F. Wojciechowski, et al., J. Chromatogr. B, 525, 240 – 245 (1990).
S. S. Good, D. J. Reynolds, and P. D. Miranda, J. Chromatogr. B, 431, 122 – 133 (1988).
Int. Conf. on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use: Q2 (R1), Validation of Analytical Procedure Text and Methodology, November 2005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garg, A., Kumar Soni, L., Kaskhedikar, S.G. et al. Development and validation of HPLC method for analysis of some antiretroviral agents in their pharmaceutical dosage forms. Pharm Chem J 43, 369–374 (2009). https://doi.org/10.1007/s11094-009-0305-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-009-0305-0