Abstract
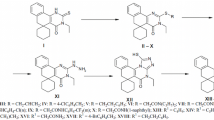
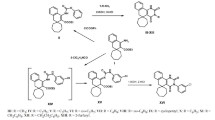
The interaction of 3-methyl-3-ethyl-1-amino-2-ethoxycarbonyl-3,4-dihydronaphthaline with phenyl-and phenethylisothiocyanates and subsequent treatment of the reaction mix with alkali led to the formation of the corresponding 3-phenyl-and 3-phenethyl-5-methyl-5-ethyl-4-oxo-2-thioxo-1,2,3,4,5,6-hexahydrobenzo[h]quinazolines (II, III). Condensation of the resulting 2-thioxobenzo[h]quinazolines II and III with halogenides of different structures was used to synthesize 2-sulfanyl-substituted 5-methyl-5-ethyl-4-oxo-3,4,5,6-tetrahydrobenzo[h]quinazolines (IV–XXVII). Reaction of benzo[h]quinazoline II with 2-ethanolamine and 3-propanolamine yielded 5-methyl-5-ethyl-3-phenyl-2-(β-hydroxyethylamino)-and 5-methyl-5-ethyl-3-phenyl-2-(γ-hydroxypropylamino)-4-oxo-3,4,5,6-tetrahydrobenzo[h]quinazolines (XXVIII, XXIX) respectively. The effects of the resulting compounds on brain monoamine oxidase (MAO) activity were studied in in vitro experiments. Most of the compounds were found to inhibit 5-HT deamination. The antitumor activities of these compounds were studied using two models of grafted mouse tumors — Ehrlich ascites carcinoma (EAC) and sarcoma 180. Some of the study compounds had moderate therapeutic effects (suppressing tumor growth by 50–60%, p < 0.05).
Similar content being viewed by others
References
O. Bruno, S. Schenone, A. Ranise, et al., Farmaco, 54, 95–100 (1999).
K. Sasaki, Y. Sekiya, H. Fujiwara, et al., J. Het. Chem., 30, 993–995 (1993).
K. Takaji, H. Hideki, T. Hirota, et al., Chem. Pharm. Bull., 23, 2015–2018 (1985); Chem. Abstr., 84, 5232w (1976).
T. Hirota, K. Kawanishi, K. Sasaki, et al., J. Het. Chem., 23, No. 3, 685–688 (1986).
T. Hirota, K. Kawanishi, and K. Sasaki, Heterocycles, 24, No. 4, 1119–1130 (1986).
R. A. Kuroyan, A. I. Markosyan, M. G. Oganisyan, et al., A.s. 1672728 (USSR).
A. I. Markosyan, R. A. Kuroyan, M. G. Oganisyan, et al., Khim.-Farm. Zh., 25, No. 6, 18–21 (1991).
A. I. Markosyan, M. G. Oganisyan, R. A. Kuroyan, et al., Khim.-Farm. Zh., 25, No. 8, 26–28 (1991).
A. I. Markosyan, Kh. S. Akopyan, B. T. Garibdzhanyan, Proceedings of the Second International Conference, “Chemistry and Biological Activity of Oxygen-and Sulfur-Containing Heterocycles [in Russian], Moscow (2003), Vol. 2, p. 143.
R. R. Safrazbekyan and R. S. Sukasyan, Vopr. Med. Khimii, 16, 623–628 (1970).
G. N. Pershin, Methods of Experimental Chemotherapy [in Russian], Medgiz, Moscow (1971).
Z. P. Sofina, A. B. Syrkin, A. Goldin, et al., Experimental Assessment of Antitumor Substances in the USSR and USA [in Russian], Meditsina, Moscow (1980).
Author information
Authors and Affiliations
Additional information
__________
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 42, No. 6, pp. 7–11, June, 2008.
Rights and permissions
About this article
Cite this article
Markosyan, A.I., Akalyan, K.S., Arsenyan, F.G. et al. Synthesis and biological properties of 3-phenyl-and 3-phenethyl-5-methyl-5-ethyl-4-oxo-3,4,5,6-tetrahydrobenzo[h]quinazolines. Pharm Chem J 42, 313–318 (2008). https://doi.org/10.1007/s11094-008-0115-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-008-0115-9