Abstract
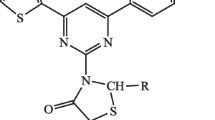
A series of pseudo-thiohydantoins and their 5-arylidene derivatives have been synthesized. Reactions of acid hydrolysis of pseudo-thiohydantoins with the formation of the corresponding 5-arylidene-3-β-aminothiazolid-2,4-one hydrochlorides have been performed. The antifungal activity of the synthesized compounds has been evaluated.
Similar content being viewed by others
References
M. D. Mashkovskii, Drugs [in Russian], Meditsina, Moscow (1993), Vol. 1.
N. N. Mel’nikov, Pesticides: Chemistry, Technology and Usage [in Russian], Khimiya, Moscow (1987).
V. V. Mozolis and S. P. Iokubaitite, Usp. Khim., 42(2), 1310–1311 (1973).
N. M. Turkevich and O. F. Lymar, Zh. Org. Khim., 31(5), 1635–1637 (1961).
T. S. Zhivotova, A. M. Gazaliev, and Z. K. Aitpaeva, Izv. Nat. Akad. Nauk Rep. Kazakh., No. 4, 31–33 (2004).
E. I. Andreeva, S. S. Kukalenko, T. S. Pronchenko, et al., Methodological Recommendations on the Determination of fungicidal Activity of New Compounds [in Russian], Cherkassy (1984).
Author information
Authors and Affiliations
Additional information
__________
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 40, No. 10, pp. 17–19, October, 2006.
Rights and permissions
About this article
Cite this article
Bakbardina, O.V., Nurmagambetova, R.T., Gazalieva, M.A. et al. Synthesis and fungicidal activity of pseudo-thiohydantoins, their 5-arylidene derivatives, and 5-arylidene-3-β-aminothiazolid-2,4-one hydrochlorides. Pharm Chem J 40, 537–539 (2006). https://doi.org/10.1007/s11094-006-0187-3
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11094-006-0187-3