Abstract
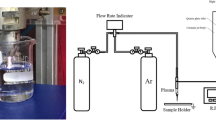
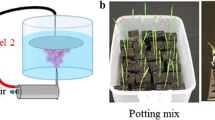
A large-volume atmospheric glow discharge has been used to incorporate nitrogen species into aqueous solution that is subsequently used to fertilize radishes, marigolds, and tomatoes. Treatment with plasma activated water (PAW) was compared to a tap water control. Water application began immediately after seeding and continued for 4 weeks. At the conclusion of the experiment, PAW treated plants had shoot masses 1.7–2.2 times larger than controls. Subsequent research has focused on optimizing the amount of nitrogen in solution by varying discharge power, air flow, air–water interface, and water alkalinity. When the discharge hovers over a stationary water phase, nitrate concentrations increase with decreasing power and increasing air flow. When water droplets are injected directly into the plasma, nitrate concentrations increase with increasing power and decreasing air flow. These contrasting trends are believed to depend on the balance between hydroxyl and electron concentrations in the discharge. Adding NaHCO3 to water before plasma treatment affects both the amount of nitrogen present in solution as well as the ratio between oxidized and reduced species. Concentrated NaHCO3 solution had 2.9 times more total nitrogen, 59 times more nitrite, and 27 % less nitrate after plasma treatment than a solution with no NaHCO3. Two factors could contribute to the spike in nitrite and decrease in nitrate: reaction of nitric and nitrous oxides with bicarbonate to form nitrite, and a decrease in disproportionation of nitrate that occurs readily at acidic pH but negligibly under neutral conditions.























Similar content being viewed by others
References
Kogelschatz U (2004) Atmospheric-pressure plasma technology. Plasma Phys Control Fusion 46.12B:B63–B75
Bakken JA (1994) High temperature processing and numerical modelling of thermal plasmas in Norway. Pure Appl Chem 66(6):1239–1246
Lelievre J, Dubreuil N, Brisset J-L (1995) Electrolysis processes in D.C. corona discharges in humid air. J Phys III 5(4):447–457
Locke BR, Sato M, Sunka P, Hoffmann MR, Chang J-S (2006) Electrohydraulic discharge and nonthermal plasma for water treatment. Ind Eng Chem Res 45(3):882–905
Traylor MJ, Pavlovich MJ, Karim S, Hait P, Sakiyama Y, Clark DS, Graves DB (2011) Long-term antibacterial efficacy of air plasma-activated water. J Phys D Appl Phys 44(47):472001
Byrns B, Wooten D, Lindsay A, Shannon S (2012) A VHF driven coaxial atmospheric air plasma: electrical and optical characterization. J Phys D Appl Phys 45:195–204
Sera B et al (2012) How various plasma sources may affect seed germination and growth. In: 2012 IEEE 13th international conference on optimization of electrical and electronic equipment (OPTIM)
Bormashenko E et al (2012) Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci Rep 2
Zhou Z et al (2011) Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agric Sci 2.1
Huang M, Yin M (2010) Effect of radio frequency discharge plasma on wheat’s seed germination and seedling growth. J Shanxi Agric Univ (Natural Science Edition) 5:006
Filatova I et al (2012) Fungicidal effects of plasma and radio-wave pre-treatments on seeds of grain crops and legumes. In: Plasma for bio-decontamination, medicine and food security. Springer, Netherlands, pp 469–479
Takaki K et al (2013) Improvements in plant growth rate using underwater discharge. J Phys Conf Ser 418(1):1–7
Park DP et al (2013) Reactive nitrogen species produced in water by non-equilibrium plasma increase plant growth rate and nutritional yield. Curr Appl Phys 13:S19–S29
Moussa D, Abdelmalek F, Benstaali B, Addou A, Hnatiuc E, Brisset J-L (2005) Acidity control of the gliding arc treatments of aqueous solutions: application to pollutant abatement and biodecontamination. Eur Phys J Appl Phys 29(2):189–199
Brisset JL, Hnatiuc E (2012) Peroxynitrite: a re-examination of the chemical properties of non-thermal discharges burning in air over aqueous solutions. Plasma Chem Plasma Process 32:655–674
Benjamin MM (2014) Water chemistry. McGraw-Hill, Boston, pp 270–272
Greenwood NN, Earnshaw A (1984) Chemistry of the elements. Pergamon, Oxford
Burlica R, Grim RG, Shih K-Y, Balkwill D, Locke BR (2010) Bacteria inactivation using low power pulsed gliding arc discharges with water spray. Plasma Process Polym 7(8):640–649
Lieberman MA, Lichtenberg AJ (1994) Principles of plasma discharges and materials processing. Wiley, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindsay, A., Byrns, B., King, W. et al. Fertilization of Radishes, Tomatoes, and Marigolds Using a Large-Volume Atmospheric Glow Discharge. Plasma Chem Plasma Process 34, 1271–1290 (2014). https://doi.org/10.1007/s11090-014-9573-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-014-9573-x