Abstract
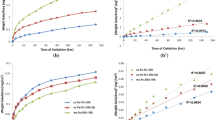
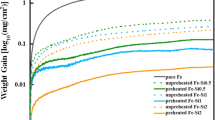
Applications at elevated temperature require alloys that can form a protective oxide layer, and Cr and Si are known to form these layers, allowing FeCrSi alloys to be oxidation resistant. However, few studies have considered Si content higher than 2 wt.%. The objective here was to analyze the cyclic oxidation resistance of innovative FeSiCrNi alloys with different Si and Cr contents (around 5 wt.% of both). Cyclic oxidation tests were performed on three alloys at 950 °C in still air. The microstructure of the cast and heat-treated alloys and the oxide scales formed were analyzed by scanning electron microscope and energy-dispersive X-ray spectroscopy. Oxide layers were also characterized by X-ray diffraction. Comparing silicon contents of 4.65 and 5.9 wt.% in two different alloys containing around 4 wt.% chromium and 0.8 wt.% carbon, it was demonstrated that the lower silicon content was not enough to avoid iron oxides formation and continuous spallation. On the other hand, the higher silicon alloy exhibited low mass gains and a protective oxide layer composed of Cr2O3 and MnCr2O4, with some enrichment of silicon at metal/oxide interface. A third alloy, containing 2.7 wt.% carbon and, thus, graphite in its microstructure suffered decarburization in some regions, although in other regions it formed a protective oxide even in an alloy containing 3.25 wt% Cr and 6.6 wt.% Si. These results demonstrate that it is possible to have a high-temperature resistant alloy with low Cr content relative to conventional alloys by using high Si additions.













Similar content being viewed by others
Availability of data and material
Data will be made available on request.
References
Q. Q. Guo, S. Liu, X. F. Wu, L. L. Liu, and Y. Niu, Scaling behavior of two Fe-xCr-5Si alloys under high and low oxygen pressures at 700°C. Corrosion Science 100, 2015 (579–588).
A. Atkinson, Transport processes during the growth of oxide films at elevated temperature. Reviews of Modern Physics 57, 1985 (437–470).
H. Wu, D. Wang, P. C. Zhang, J. M. Liang, S. Liu, and D. Tang, Influences of alloying elements on oxidation behavior of steels and microstructure of oxide scales. Journal of Iron and Steel Research International 23, 2016 (231–237).
K. Kusabiraki, R. Watanabe, T. Ikehata, M. Takeda, T. Onishi, and X. Guo, High-temperature oxidation behavior and scale morphology of Si-containing steels. ISIJ International 47, 2007 (1329–1334).
M. Fukumoto, S. Maeda, S. Hayashi, and T. Narita, Effect of water vapor on the oxidation behavior of Fe-1.5Si in air at 1073 and 1273 K. Oxidation of Metals 55, 2001 (401–422).
T. Nishimoto, K. Honda, Y. Kondo, and K. Uemura, Effects of Si content on the oxidation behavior of Fe-Si alloys in air. Materials Science Forum 696, 2011 (126–131).
L. L. Liu, Q. Q. Guo, and Y. Niu, Transition between different oxidation modes of binary Fe-Si alloys at 600–800°C in pure O2. Oxidation of Metals 79, 2013 (201–224).
A. R. Lashin, Oxidation of silicon from an Fe-6 at% Si alloy. Journal of Alloys and Compounds 567, 2013 (54–58).
M. A. A. Motin, J. Zhang, P. R. Munroe, and D. J. Young, Internal oxidation and metal dusting of Fe-Si alloys. Corrosion Science 52, 2010 (3280–3286).
T. Adachi and G. H. Meier, Oxidation of Iron-Silicon Alloys. Oxidation of Metals 27, 1987 (347–366).
Kučera J, Hajduga M. High temperature and long time oxidation of iron and steels. Wydaw. PŁ Filia W Bielsk. p. 15 (1998).
D. T. Hoelzer, B. A. Pint, and I. G. Wright, A microstructural study of the oxide scale formation on ODS Fe - 13Cr steel. Journal of Nuclear Materials 283–287, 2000 (1306–1310).
M. H. Huan, X. Qi, J. Cao, A. L. Cong, and Y. Y. Tao, Effect of Si on high temperature oxidation of 30Cr13 stainless steel. Journal of Iron and Steel Research International 24, 2017 (561–568).
J. S. Dunning, D. E. Alman, and J. C. Rawers, Influence of silicon and aluminum additions on the oxidation resistance of a lean-chromium stainless steel. Oxidation of Metals 57, 2002 (409–425).
H. E. Evans, D. A. Hilton, R. A. Holm, and S. J. Webster, Influence of silicon additions on the oxidation resistance of a stainless steel. Oxidation of Metals 19, 1983 (1–18).
A. M. Huntz, V. Bague, G. Beauplé, et al., Effect of silicon on the oxidation resistance of 9% Cr steels. Applied Surface Science 207, 2003 (255–275).
G. H. Meier, K. Jung, N. Mu, and N. M. Yanar, Effect of Alloy Composition and Exposure Conditions on the Selective Oxidation Behavior of Ferritic Fe – Cr and Fe – Cr – X Alloys. Oxidation of Metals 74, (5), 2010 (319–340).
J. Moon, S. Kim, W. D. Park, et al., Initial oxidation behavior of Fe-Cr-Si alloys in 1200 °C steam. Journal of Nuclear Materials 513, 2019 (297–308).
D. B. V. Castro, L. S. Rossino, A. M. S. Malafaia, M. Angeloni, and O. Maluf, Influence of annealing heat treatment and Cr, Mg, and Ti alloying on the mechanical properties of high-silicon cast iron. Journal of Materials Engineering and Performance 20, 2011 (1346–1354).
A. T. Kuhn, D. Wakeman, E. Y. El Roubi, and G. C. S. Collins, Anodic dissolution and oxygen evolution on binary and ternary iron-silicon alloys. Electrochimica Acta 28, 1983 (515–527).
Y. Zhang, X. Jun, Y. Zhang, et al., The study on corrosion behavior and corrosion resistance of ultralow carbon high silicon iron-based alloy. Materials Research Express 2021. https://doi.org/10.1088/2053-1591/abdc52.
D. Ding, Y. Zhang, X. Yu, et al., Effects of environmental factors on corrosion behavior of high-silicon cast iron in Shanxi soil medium. Anti-Corrosion Methods Materials 65, 2018 (538–546).
B. H. Kim, J. S. Shin, S. M. Lee, and B. M. Moon, Improvement of tensile strength and corrosion resistance of high-silicon cast irons by optimizing casting process parameters. Journal of Materials Science 42, 2007 (109–117).
A. M. S. de Malafaia. Oxidação cíclica em alta temperatura de ligas de alta entropia. D. Sc. thesis [in Portuguese]. Universidade de São Paulo, (2013).
A. M. S. Malafaia, M. T. Milan, M. Omar, R. M. Muñoz Riofano, and M. F. De Oliveira, Oxidation and abrasive wear of Fe-Si and Fe-Al intermetallic alloys. Journal of Materials Science 45, 2010 (5393–5397).
A. Atkinson, A theoretical analysis of the oxidation of FeSi alloys. Corrosion Science 22, 1982 (87–102).
V. F. de Souza, A. J. Araújo, J. L. N. do Santos, R. C. A. Della, and A. M. de Sousa Malafaia, Kinetics oxidation and characterization of cyclically oxidized layers at high temperatures for FeMnSiCrNiCe and FeSiCrNi alloys. Materials Research 20, 2017 (365–373).
R. Y. Chen and W. Y. D. Yuen, Review of the high-temperature oxidation of iron and carbon steels in air or oxygen. Oxidation of Metals 59, 2003 (433–468).
R. N. Durham, B. Gleeson, and D. J. Young, Factors affecting chromium carbide precipitate dissolution during alloy oxidation. Oxidation of Metals 50, 1998 (139–165).
S. Vazehrad, J. Elfsberg, and A. Diószegi, Study of microstructure and silicon segregation in cast iron using color etching and electron microprobe analysis. Materials Characterization 104, 2015 (132–138).
D. M. Stefanescu, G. Alonso, P. Larrañaga, and R. Suarez, On the stable eutectic solidification of iron-carbon-silicon alloys. Acta Materials 103, 2016 (103–114).
G. Rivera, R. Boeri, and J. Sikora, Revealing and characterising solidification structure of ductile cast iron. Materials Science and Technology 18, 2002 (691–697).
G. L. Rivera, R. E. Boeri, and J. A. Sikora, Solidification of gray cast iron. Scripta Materialia 50, 2004 (331–335).
S. Daniele,A. M. S. de Malafaia. Análise de parâmetros em ensaios de oxidação cíclica. COEN - Congresso de Engenharias (Engineering Congress). p. 20 (2018).
V. G. Efremenko, Y. G. Chabak, A. Lekatou, A. E. Karantzalis, and A. V. Efremenko, High-temperature oxidation and decarburization of 1455 wt pct Cr-cast iron in dry air atmosphere. Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science 47, 2016 (1529–1543).
D. Jedrzejczyk, M. Hajduga, and R. Lorek. High temperature oxidation as the method of surface treatment of cast iron. 17th International Metallurgical and Materials Conference Met. 2008 - Proceedings. (2008). pp. 1–6.
T. L. Baum, R. J. Fruehan, and S. Sridhar, Kinetics of oxidation and decarburization in Al-Si transformation induced plasticity steel. Metallurgical and Materials Transactions B Metallurgy and Materials Processing Science 38, 2007 (287–297).
C. H. Yang, S. N. Lin, C. H. Chen, and W. T. Tsai, Effects of temperature and straining on the oxidation behavior of electrical steels. Oxidation of Metals 72, 2009 (145–157).
C. M. Chun and T. A. Ramanarayanan, Corrosion resistance of a high-silicon alloy in metal-dusting environments. Oxidation of Metals 67, 2007 (215–234).
N. Israelsson, K. A. Unocic, K. Hellström, et al., A Microstructural and Kinetic Investigation of the KCl-Induced Corrosion of an FeCrAl Alloy at 600 °C. Oxidation of Metals 84, 2015 (105–127).
Z. Żurek, A. Jaroń, and M. Homa, Morphology analysis of the scale formed on Crofer 22APU steel in atmospheres containing SO2. Oxidation of Metals 76, 2011 (273–285).
Acknowledgements
The authors would like to thank CAPES (Coordination of Superior Level Staff Improvement) for providing master scholarship.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pereira, J.N., de Souza, V.F. & De Sousa Malafaia, A.M. Evaluation of Si Content in FeSiCrNi Alloys Containing Carbon on Cyclic Oxidation Resistance at 950 °C. Oxid Met 96, 453–468 (2021). https://doi.org/10.1007/s11085-021-10035-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-021-10035-w