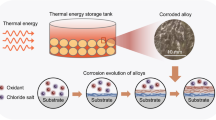
Abstract
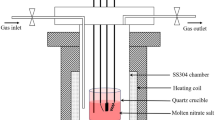
Solar salt (60 wt% NaNO3 + 40 wt% KNO3) is widely used in the storage system of concentrated solar power plant. However, the chloride within the molten solar salt can affect the corrosion kinetics of stainless steels and hence affect the overall structural integrity. In this work, the corrosion behavior of 304 and 316L stainless steel (SS) in molten solar salt mixtures with different chloride impurities were investigated by immersion tests at 565 °C. Results show that as the content of chloride in the solar salt increases (up to 1.4 wt%), both 304 and 316L SS keep parabolic corrosion kinetics, and the rate constants increase. In comparison with 304 SS, the better corrosion resistance of 316L SS is ascribed to the higher thickness ratio of FeCr2O4 spinel to oxide scale. In addition, to quantitatively describe the effect of chloride impurity on corrosion kinetics of stainless steels, a novel theoretical model based on Fick’s first law is proposed and validated by the experiments.














Similar content being viewed by others
References
F. P. G. Márquez, A. Karyotakis, and M. Papaelias, Renewable energies: business outlook 2050, (Springer, Berlin, 2018).
T. E. Boukelia, A. Ghellab, A. Laouafi, A. Bouraoui, and Y. Kabar, Cooling performances time series of CSP plants: calculation and analysis using regression and ANN models. Renewable Energy 157, 2020 (809–827).
W. Gregory, Liquid Metal Based High Temperature Concentrated Solar Power: Cost Considerations. Diss. (Georgia Institute of Technology, 2016).
M. Kasra and H. Khorasanizadeh, The potential and deployment viability of concentrated solar power (CSP) in Iran. Energy Strategy Reviews 24, 2019 (358–369).
C. Emiliano, F. Casella, and P. Colonna, Design of CSP plants with optimally operated thermal storage. Solar Energy 116, 2015 (371–387).
P. Ugo, L. Luo, Y. Fan, D. Stitou, and M. Rood, Thermal energy storage systems for concentrated solar power plants. Renewable Sustainable Energy Reviews 79, 2017 (82–100).
A. G. Fernández, M. Fullana, L. Calabrese, V. Palomba, A. Frazzica, and L. F. Cabeza, Corrosion assessment of promising hydrated salts as sorption materials for thermal energy storage systems. Renewable Energy 150, 2020 (428–434).
A. Bonk, C. Martin, M. Braun, T. Bauer, Material investigations on the thermal stability of solar salt and potential filler materials for molten salt storage, in AIP Conference Proceedings, Vol. 1850 (2017), No. 1, p. 080008.
A. Bonk, S. Sau, N. Uranga, M. Hernaiz, and T. Bauer, Advanced heat transfer fluids for direct molten salt line-focusing CSP plants. Progress in Energy and Combustion Science 67, 2018 (69–87).
R. I. Olivares, The thermal stability of molten nitrite/nitrates salt for solar thermal energy storage in different atmospheres. Solar Energy 86, (9), 2012 (2576–2583).
D. Kearney, U. Herrmann, P. Nava, B. Kelly, R. Mahoney, J. Pacheco, et al., Assessment of a molten salt heat transfer fluid in a parabolic trough solar field. Journal of Solar Energy Engineering 125, (2), 2003 (170–176).
K. Vignarooban, X. Xu, A. Arvay, K. Hsu, and A. M. Kannan, Heat transfer fluids for concentrating solar power systems—a review. Applied Energy 146, 2015 (383–396).
D. A. Nissen and D. E. Meeker, Nitrate/nitrite chemistry in sodium nitrate-potassium nitrate melts. Inorganic Chemistry 22, (5), 1983 (716–721).
C. Villada, A. Bonk, T. Bauer, and F. Bolívar, High-temperature stability of nitrate/nitrite molten salt mixtures under different atmospheres. Applied Energy 226, 2018 (107–115).
R. W. Bradshaw and D. E. Meeker, High-temperature stability of ternary nitrate molten salts for solar thermal energy systems. Solar Energy Material and Solar Cell 21, (1), 1990 (51–60).
K. H. Stern, High temperature properties and decomposition of inorganic salts part 3, nitrates and nitrites. Journal of Physical and Chemical Reference Data 1, (3), 1972 (747–772).
Q. Peng, X. Wei, J. Ding, J. Yang, and X. Yang, High—temperature thermal stability of molten salt materials. International Journal of Energy Research 32, (12), 2008 (1164–1174).
X. Wei, Y. Wang, Q. Peng, J. Yang, X. Yang, and J. Ding, NOx emissions and NO2-formation in thermal energy storage process of binary molten nitrate salts. Energy 74, 2014 (215–221).
V. A. Sötz, A. Bonk, J. Steinbrecher, and T. Bauer, Defined purge gas composition stabilizes molten nitrate salt-experimental prove and thermodynamic calculations. Solar Energy 211, 2020 (453–462).
A. Baraka, A. I. Abdel-Rohman, and A. E. Hosary, Corrosion of mild steel in molten sodium nitrate–potassium nitrate eutectic. British Corrosion Journal 11, (1), 1976 (44–46).
M. Zhu, S. Zeng, H. Zhang, J. Li, and B. Cao, Electrochemical study on the corrosion behaviors of 316 SS in HITEC molten salt at different temperatures. Solar Energy Materials and Solar Cells 186, 2018 (200–207).
R. W. Bradshaw, S. H. Goods, Corrosion resistance of stainless steels during thermal cycling in alkali nitrate molten salts. No. SAND2001-8518. (SANDIA National Laboratories, 2001).
A. G. Fernández, M. I. Lasanta, and F. J. Pérez, Molten salt corrosion of stainless steels and low-Cr steel in CSP plants. Oxidation of Metals 78, (5–6), 2012 (329–348).
A. Kruizenga and D. Gill, Corrosion of iron stainless steels in molten nitrate salt. Energy Procedia 49, 2014 (878–887).
R. W. Bradshaw, W. M. Clift, Effect of chloride content of molten nitrate salt on corrosion of A516 carbon steel. Sandia report (2010).
W. Magdalena, F. Pineda, A. G. Fernández, C. Mata-Torres, and R. A. Escobar, Materials corrosion for thermal energy storage systems in concentrated solar power plants. Renewable Sustainable Energy Reviews 86, 2018 (22–44).
S. Bell, T. Steinberg, and G. Will, Corrosion mechanisms in molten salt thermal energy storage for concentrating solar power. Renewable Sustainable Energy Reviews 114, 2019 (109328).
J. Zhang, Y. LeYann, A systematic comparison of supercritical CO2 Brayton cycle layouts for concentrated solar power with a focus on thermal energy storage utilization, in 3rd European Conference on Supercritical CO2 (sCO2) Power Systems 2019, 19th–20th September 2019, p. 105–115.
A. G. Fernández and L. F. Cabeza, Molten salt corrosion mechanisms of nitrate based thermal energy storage materials for concentrated solar power plants: a review. Solar Energy Materials and Solar Cells 194, 2019 (160–165).
M. T. Islam, N. Huda, and R. Saidur, Current energy mix and techno-economic analysis of concentrating solar power (CSP) technologies in Malaysia. Renewable Energy 140, 2019 (789–806).
R. Moore, M. Vernon, C. K. Ho, N. P. Siegel, G. Kolb, Design considerations for concentrating solar power tower systems employing molten salt, Sandia National Laboratories report: SAND2010-6978 (2010).
M. Papaelias, L. Cheng, M. Kogia, A. Mohimi, V. Kappatos, C. Selcu, L. Constantinou, M. Gómez, Q. Carlos, M. Garcia, P. Fausto, and T. H. Gan, Inspection and structural health monitoring techniques for concentrated solar power plants. Renewable Energy 85, 2016 (1178–1191).
S. H. Goods, R. W. Bradshaw, M. R. Prairie, J. M. Chavez, Corrosion of stainless and carbon steels in molten mixtures of industrial nitrates, No. SAND-94-8211. Sandia National Labs., Livermore, CA (United States) (1994).
A. S. Dorcheh, R. N. Durham, and M. C. Galetz, High temperature corrosion in molten solar salt: the role of chloride impurities. Materials and Corrosion 68, (9), 2017 (943–951).
A. Gomes, M. Navas, N. Uranga, T. Paiva, I. Figueira, and T. C. Diamantino, High-temperature corrosion performance of austenitic stainless steels type AISI 316L and AISI 321H, in molten Solar Salt. Solar Energy 177, 2019 (408–419).
A. Bonk, D. Rückle, S. Kaesche, M. Braun, and T. Bauer, Impact of Solar Salt aging on corrosion of martensitic and austenitic steel for concentrating solar power plants. Solar Energy Materials and Solar Cells 203, 2019 (110162).
D. Fähsing, C. Oskay, T. M. Meißner, and M. C. Galetz, Corrosion testing of diffusion-coated steel in molten salt for concentrated solar power tower systems. Surface and Coatings Technology 354, 2018 (46–55).
C. Prieto, J. Gallardo-González, F. J. Ruiz-Cabañas, C. Barreneche, M. Martínez, M. Segarra, and A. I. Fernández, Study of corrosion by Dynamic Gravimetric Analysis (DGA) methodology. Influence of chloride content in solar salt. Solar Energy Materials and Solar Cells 157, 2016 (526–532).
American Society for Testing and Materials (Filadelfia, Pennsylvania), ASTM G1-03: Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. (ASTM, 2004).
W. J. Tobler and S. Virtanen, Effect of Mo species on metastable pitting of Fe18Cr alloys—a current transient analysis. Corrosion Science 48, (7), 2016 (1585–1607).
C. R. Clayton and Y. C. Lu, A bipolar model of the passivity of stainless steel: the role of Mo addition. Journal of the Electrochemical Society 133, (12), 1986 (2465).
W. D. Robertson, Molybdate and tungstate as corrosion inhibitors and the mechanism of inhibition. Journal of the Electrochemical Society 98, (3), 1951 (94).
A. Pardo, M. C. Merino, A. E. Coy, F. Viejo, R. Arrabal, and E. J. C. S. Matykina, Pitting corrosion behaviour of austenitic stainless steels–combining effects of Mn and Mo additions. Corrosion Science 50, (6), 2008 (1796–1806).
A. S. Dorcheh, R. N. Durham, and M. C. Galetz, Corrosion behavior of stainless and low-chromium steels and IN625 in molten nitrate salts at 600 C. Solar Energy Materials and Solar Cells 144, 2016 (109–116).
K. L. Summers and D. Chidambaram, Corrosion behavior of structural materials for potential use in nitrate salts based solar thermal power plants. Journal of The Electrochemical Society 164, (8), 2017 (H5357).
H. J. Grabke, E. Reese, and M. Spiegel, The effects of chlorides, hydrogen chloride, and sulfur dioxide in the oxidation of steels below deposits. Corrosion Science 37, (7), 1995 (1023–1043).
A. Palacios, M. E. Navarro, Z. Jiang, A. Avila, G. Qiao, E. Mura, and Y. Ding, High-temperature corrosion behaviour of metal alloys in commercial molten salts. Solar Energy 201, 2020 (437–452).
G. Y. Lai, High temperature corrosion of engineering alloys. United States: N. P. (1990).
D. A. Tillman, D. Dao, and M. Bruce, Chlorine in solid fuels fired in pulverized fuel boilers-sources, forms, reactions, and consequences: a literature review. Energy Fuels 23, (7), 2009 (3379–3391).
A. Zahs, M. Spiegel, and H. Grabke, The influence of alloying elements on the chlorine—induced high temperature corrosion of Fe–Cr alloys in oxidizing atmospheres. Materials and Corrosion 50, (10), 1999 (561–578).
S. Enestam, D. Bankiewicz, J. Tuiremo, K. Mäkelä, and M. Hupa, Are NaCl and KCl equally corrosive on superheater materials of steam boilers? Fuel. 104, 2013 (294–306).
N. Perez, Electrochemistry and Corrosion Science, (Kluwer Academic Publishers, Boston, 2004).
Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 51905261), Natural Science Foundation of Jiangsu Province (No. BK20190678), National Key R&D Program of China (No. 2018YFC0808800) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX20_1003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Yang, X., Yin, X. et al. Effect of Chloride Impurity on Corrosion Kinetics of Stainless Steels in Molten Solar Salt for CSP Application: Experiments and Modeling. Oxid Met 95, 311–332 (2021). https://doi.org/10.1007/s11085-021-10025-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-021-10025-y