Abstract
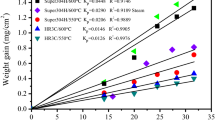
Oxidation tests on a ferritic–martensitic steel were carried out in deaerated supercritical water at 600–700 °C under 25 MPa pressure. The oxidation kinetics followed near-parabolic rate law at 600 °C and obeyed near-cubic rate law at 650–700 °C. The deviations from parabolic behaviour may have been related to the development of growth stresses within the oxide. The oxidation rate did not always increase significantly with increasing temperature. The oxidation rates at 650 and 700 °C were approximately equal. The reason for this may be attributed to the formation of large pores at the interface between oxide scale and substrate at 700 °C. The influence of temperature on the microstructure of oxide scale and oxidation kinetics is discussed.
Graphical Abstract










Similar content being viewed by others
References
R. Viswanathan, J. Sarver and J. M. Tanzosh, Journal of Materials Engineering and Performance 15, 2006 (255).
N. Q. Zhang, Z. L. Zhu, F. B. Lv, D. F. Jiang and H. Xu, Oxidation of Metals 86, 2016 (1).
H. Xu, Z. L. Zhu and N. Q. Zhang, Oxidation of Metals 82, 2014 (21).
J. Bischoff, A. Motta, C. Eichfeld, R. Comstock, G. Cao and T. Allen, Journal of Nuclear Materials 441, 2013 (604).
N. Zhang, H. Xu, B. Li, Y. Bai and D. Liu, Corrosion Science 56, 2012 (123).
Y. Chen, K. Sridharan and T. R. Allen, Corrosion Science 48, 2006 (2843).
X. Y. Zhong, X. Q. Wu and E. H. Han, Corrosion Science 90, 2015 (511).
L. Tan, X. Ren and T. R. Allen, Corrosion Science 52, 2010 (1520).
K. Yin, S. Qiu, R. Tang, Q. Zhang and L. Zhang, The Journal of Supercritical Fluids 50, 2009 (235).
J. Żurek, E. Wessel, L. Niewolak, F. Schmitz, T.-U. Kern, L. Singheiser and W. J. Quadakkers, Corrosion Science 46, 2004 (2301).
D. J. Young, J. Zurek, L. Singheiser and W. J. Quadakkers, Corrosion Science 53, 2011 (2131).
N. Q. Zhang, Z. L. Zhu, H. Xu, X. P. Mao and J. Li, Corrosion Science 103, 2016 (124).
M. Nezakat, H. Akhiani, S. Penttilä, S. M. Sabet and J. Szpunar, Corrosion Science 94, 2015 (197).
L. Tan, M. T. Machut, K. Sridharan and T. R. Allen, Journal of Nuclear Materials 371, 2007 (161).
L. Tan, Y. Yang and T. R. Allen, Corrosion Science 48, 2006 (3123).
X. Ren, K. Sridharan and T. R. Allen, Journal of Nuclear Materials 358, 2006 (227).
J. Stringer, Corrosion Science 10, 1970 (513).
J. Bischoff and A. T. Motta, Journal of Nuclear Materials 424, 2012 (261).
Z. L. Zhu, H. Xu, D. F. Jiang, G. Q. Yue, B. R. Li and N. Q. Zhang, The Journal of Supercritical Fluids 10, 2016 (56).
R. Haugsrud, Corrosion Science 45, 2003 (211).
H. E. Evans, D. J. Norfolk and T. Swan, Journal of The Electrochemical Society 125, 1978 (1180).
H. E. Evans, International Materials Reviews 40, 1995 (1).
W. J. Quadakkers, Materials and Corrosion 41, 1990 (659).
D. Naumenko, B. Gleeson, E. Wessel, L. Singheiser and W. J. Quadakkers, Metallurgical and Materials Transactions A 38, 2007 (2974).
Z. Liu, W. Gao and Y. He, Oxidation of Metals 341, 2000 (53).
L. Martinelli, F. Balbaud-Célérier, A. Terlain, S. Delpech, G. Santarini, J. Favergeon, G. Moulin, M. Tabarant and G. Picard, Corrosion Science 50, 2008 (2537).
N. B. Pilling and R. E. Bedworth, Journal Institute of Metals 29, 1923 (529–582).
D. R. Clarke, Acta Materialia 51, 2003 (1393).
A. M. Limarga, D. S. Wilkinson and G. C. Weatherly, Scripta Materialia 50, 2004 (1475).
F. N. Rhines and J. S. Wolf, Metallurgical and Materials Transactions 1, 1970 (1701).
B. Pieraggi and R. A. Rapp, Acta Metallurgica 36, 1988 (1281).
J. Robertson, Corrosion Science 32, 1991 (443).
P. Kofstad, Oxidation of Metals 24, 1985 (265).
A. Atkinson and D. W. Smart, Journal of The Electrochemical Society 135, 1988 (2886).
L. Martinelli, F. Balbaud-Célérier, A. Terlain, S. Delpech, G. Santarini, J. Favergeon, G. Moulin, M. Tabarant and G. Picard, Corrosion Science 50, 2008 (2523).
D. J. Young, High Temperature Oxidation and Corrosion of Metals. in Corrosion Series, vol. 1, ed. T. Burstein (Elsevier Science, Amsterdam, 2008).
I. G. Wright, J. Y. Howe and A. S. Sabau, Materials at High Temperatures 26, 2009 (105).
W. Christl, A. Rahmel and M. Schütze, Oxidation of Metals 31, 1989 (35).
V. K. Tolpygo, J. R. Dryden and D. R. Clarke, Acta Materialia 46, 1998 (927).
M. V. Speight and J. E. Harris, Acta Metallurgica 26, 1978 (1043).
P. Ampornrat and G. S. Was, Journal of Nuclear Materials 371, 2007 (1).
W. J. Quadakkers, P. J. Ennis, J. Żurek and M. Michalik, Materials at High Temperatures 22, 2005 (47).
R. J. Ehlers, E. J. Smaardijk, H. J. Penkala, A. K. Tyagi, L. Singheiser, W. J. Quadakkers, Effect of steel composition on the bell-shape temperature dependence of oxidation in water vapour containing environments, in: Proceedings of the International Corrosion Congress, Cape Town, Proceedings, Paper 336.
J. Żurek, M. Michalik, F. Schmitz, T.-U. Kern, L. Singheiser and W. J. Quadakkers, Oxidation of Metals 63, 2005 (401).
H. E. Evans, Materials Science and Technology 4, 1988 (1089).
L. Tomlinson and N. J. Cory, Corrosion Science 29, 1989 (939).
Acknowledgments
This paper was supported by National Natural Science Foundation of China (51471069), Natural Science Foundation of Beijing (2152029) and the Fundamental Research Funds for the Central Universities (2015ZZD05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Z., Xu, H., Jiang, D. et al. Temperature Dependence of Oxidation Behaviour of a Ferritic–Martensitic Steel in Supercritical Water at 600–700 °C. Oxid Met 86, 483–496 (2016). https://doi.org/10.1007/s11085-016-9647-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-016-9647-7