Abstract
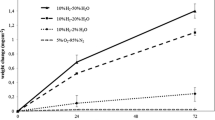
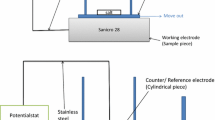
The effect of KCl on the corrosion behavior of the austenitic stainless steel 304L was studied at 600 °C in 5% O2 + 40% H2O + N2. The breakdown of the protective oxide was investigated. This was done through a detailed microstructural characterization of the oxide scales formed after 1, 24 and 168 h. The oxidized samples were investigated by SEM/EDX, FIB and STEM/EDX. The presence of KCl(s) causes a breakdown of most of the protective scale, even though it is not in direct contact with KCl(s) particles, starting after just 1 h exposure. A fast growing porous oxide formed in direct contact with (former) KCl(s) particles and an about 2 μm thick scale covered most of the surface. Only some regions were covered by a thin scale. K2CrO4 particles were randomly distributed all over the scale after 1 h exposure. The particles are situated above the oxide scale and are not in direct contact with the subjacent metal. The thin scale contains lower Cr levels than has been observed in corresponding scales formed in the absence of KCl. The breakdown of the protective scale is suggested to be caused primarily by the formation of K2CrO4, depleting the protective oxide in chromium. In addition, chromia evaporation contributes to chromia depletion and breakdown of the protective scale. Very little or no transition metal chlorides were found after breakaway oxidation. Cl is suggested to play a minor role in the initial breakdown of the protective scale. The presence of KCl particles caused local rapid oxidation, which results in an outward growing Fe and Fe–Cr rich porous oxide.
























Similar content being viewed by others
References
H. Asteman, et al., Oxidation of Metals 52, 95 (1999).
H. Asteman, et al., Oxidation of Metals 54, 11 (2000).
J. Pettersson, et al., Oxidation of Metals 64, 23 (2005).
C. Pettersson, J. Pettersson, H. Asteman, J.-E. Svensson, and L.-G. Johansson, Corrosion Science 48, 1368 (2006).
T. Jonsson, et al., in 16th International Corrosion Congress (Beijing, China, 2005).
H. J. Grabke, E. Reese, and M. Spiegel, Corrosion Science 37, 1023 (1995).
H. P. Michelsen, et al., Fuel Processing Technology 54, 95 (1998).
M. Montgomery and A. Karlsson, Materials and Corrosion 50, 579 (1999).
H. P. Nielsen, F. J. Frandsen, and K. Dam-Johansen, Energy & Fuels 13, 1114 (1999).
Y. Shu, F. Wang, and W. Wu, Oxidation of Metals 54, 457 (2000).
M. Spiegel, C. Schroer, and H. J. Grabke, Materials Science Forum 251–254, 527 (1997).
C. J. Wang and T. T. He, Oxidation of Metals 58, 415 (2002).
S. Y. Lee and M. J. McNallan, Corrosion 47, 868 (1991).
Y. Shinata, Oxidation of Metals 27, 315 (1987).
E. M. Levin, C. R. Robbins, and H. F. McMurdie, in Phase Diagrams for Ceramists (The American Ceramic Society Inc, 1964).
M. W. Phaneuf, Micron 30, 277 (1999).
J. E. Tang, et al., Micron 32, 799 (2001).
M. Halvarsson, et al., Corrosion Science 48, 2014 (2006).
Acknowledgements
This work was carried out within the Swedish High Temperature Corrosion Centre (HTC). A grant from the Knut and Alice Wallenberg Foundation for acquiring the FEG-SEM instruments is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jonsson, T., Froitzheim, J., Pettersson, J. et al. The Influence of KCl on the Corrosion of an Austenitic Stainless Steel (304L) in Oxidizing Humid Conditions at 600 °C: A Microstructural Study. Oxid Met 72, 213–239 (2009). https://doi.org/10.1007/s11085-009-9156-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-009-9156-z