Abstract
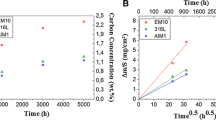
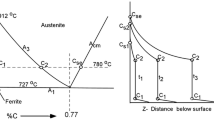
A Ni–20Cr alloy and variants containing 5, 10 and 20Cu (all in wt.%) were carburised in H2-5% CH4 at 1,000 °C. All alloys formed internal carburisation zones containing Cr3C2 and Cr7C3. The Ni–20Cr alloy also developed a surface deposit of graphite, but the copper-bearing alloys did not. Measured parabolic rate constants for intragranular carburisation were used to calculate carbon permeabilities from Wagner’s diffusion analysis. The value obtained for Ni–20Cr was in good agreement with the product of independently measured carbon solubility and diffusion coefficient values for nickel. Permeabilities found for copper-bearing alloys were similar, showing that the presence of copper had little effect on carbon diffusion in nickel. This finding is used in analysing the mechanism by which nickel undergoes metal dusting.





Similar content being viewed by others
References
C. A. Bernardo, I. Alstrup, and J. R. Rostrup-Nielsen, Journal of Catalysis 96, 517 (1985).
I. Alstrup, M. T. Tavares, C. A. Bernardo, O. Sørensen, and J. R. Rostrup-Nielsen, Materials and Corrosion 49, 367 (1998).
J. A. Dalmon and G. A. Martin, Journal of Catalysis 66, 214 (1980).
M. T. Tavares, I. Alstrup, and C. A. Bernard, Materials and Corrosion 50, 681 (1999).
J. Zhang, D. M. I. Cole, and D. J. Young, Materials and Corrosion 56, 756 (2005).
J. Zhang and D. J. Young, Corrosion Science 49, 1450 (2007).
Y. Nishiyama, K. Moriguchi, N. Otsuka, and T. Kudo, Materials and Corrosion 56, 806 (2005).
DKI German Copper Institute Booklet, Copper Nickel Alloys: Properties, Processing, Application. http://www.copper.org/applications/cuni/txt_DKI.html.
R. B. McLellan and P. Chraska, Materials Science and Engineering 6, 176 (1970).
C. Wagner, Zeitschrift Fur Elektrochemie 63, 772 (1959).
T. Wada, H. Wada, J. F. Elliott, and J. Chipman, Metal Transition 2A, 2199 (1971).
A. T. Allen and D. L. Douglass, Oxidation of Metals 51, 199 (1999).
K. Monma, H. Suto, and H. Oikawa, Nippon Kinzoku Gakkaishi 28, 188 (1964).
D. J. Young, High Temperature Oxidation and Corrosion of Metals (Elsevier, in press).
P. Villars, A. Prince, and H. Okamoto, Handbook of Ternary Alloy Phase Diagrams (ASM International, USA, 1997).
W. B. Pearson and L. T. Thompson, Canadian Journal of Physics 35, 349 (1957).
Acknowledgements
Support of this study by the Australian Research Council is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Safarzadeh, M. & Young, D.J. Carbon Permeability of Nickel and Ni–Cu Alloys. Oxid Met 70, 15–24 (2008). https://doi.org/10.1007/s11085-008-9108-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-008-9108-z