Abstract
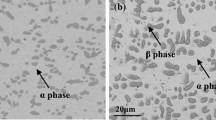
The oxidation of three Cu–xCr–2Al and three Cu–xCr–4Al alloys (x ≅ 0,4,8 at.%) has been investigated at 800°C in 1 atm O2. Oxidation of a binary Cu–Al alloy containing 2.2 at.% Al produced external scales composed mainly of copper oxides with small amounts of Al-rich oxide in the inner region, while the internal oxidation of Al was almost absent. The addition of 3.9 at.% Cr to this alloy was able to decrease the oxidation rate but was insufficient to prevent the oxidation of copper. Conversely, addition of 8.1 at.% Cr to the same binary alloy promoted the rather fast formation of a protective Al2O3 layer in contact with the metal substrate, with a simultaneous large decrease in the oxidation rate, producing a form of third-element effect. On the contrary, all the Cu–xCr–4Al alloys formed an internal Al2O3 layer after an initial stage during which all the alloy components were oxidized, so that the only effect of the presence of chromium was to decrease the duration of the fast initial stage. The third-element effect due to chromium additions to Cu–2Al is related to a transition from the formation of external scales composed of mixtures of Cu and Al oxides to the external growth of Al2O3–rich scales as a consequence of a thermodynamic destabilization of copper oxides associated with the formation of solid solutions between Al2O3 and Cr2O3.










Similar content being viewed by others
References
Dunn J. S. (1934) Journal of the Institute of Metals 46:25
Frohlich K. W. (1936) Zeitschrift for Metallkunde 28:368
Price L. E., Thomas G. J. (1938) Journal of the Institute of Metals 63:21
Dennison J. P., Preece A. (1952) Journal of the Institute of Metals 81:229
Blade J. C., Preece A. (1959) Journal of the Institute of Metals 88:427
Sanderson M. D., Scully J. C. (1971) Oxidation of Metals 3:59
Hauffe K., Ofulue E. (1972) Werkstoffe Und Korrosion – Materials and Corrosion 23:351
Massalski T. B. (1990) Binary Alloy Phase Diagrams. ASM International, Metals Park
Niu Y., Gesmundo F., Douglass D. L., Viani F. (1997) Oxidation of Metals 48:357
Gesmundo F., Viani F., Niu Y. (1994) Oxidation of Metals 42:409
Gesmundo F., Niu Y., Viani F. (1995) Oxidation Metals 43:379
Gesmundo F., Niu Y., Viani F., Douglass D. L. (1998) Oxidation of Metals 49:147
Fu G. Y., Niu Y., Gesmundo F. (2003) Corrosion Science 45:559
Villars P., Prince A., Okamoto H. (eds) (1997) Handbook of Ternary Alloy Phase Diagrams. ASM International, Metals, Park, USA
Gesmundo F., De Asmundis C., Merlo S. (1979) Werkstoffe Und Korrosion – Materials and Corrosion 30:114
Tomaszewicz P., Wallwork G. R. (1983) Oxidation of Metals 19:165
Gesmundo F., Niu Y. (1998) Oxidation Metals 50:1
Wagner C. (1959) Zeitschrift Fur Elektrochemie 63:772
Rapp R. A. (1961) Acta Metallurgica 9:730
Rapp R. A. (1965) Corrosion 21:382
Gesmundo F., Viani F. (1986) Oxidation of Metals 25:269
Maak F. (1961) Zeitschrift Fur Metallkunde 52:538
Crank J. (1965) The Mathematics of Diffusion. Clarendon Press, Oxford
Tarula M. L., Tare V. B., Worrell W. L. (1983) Metallurgical Transactions B 14B:673
Rhines F. N., Mehl R. F. (1938) Transactions of the Metallurgical Society of AIME 128:185
Hirone T., Kunitomi N., Sakamoto M., Yamaki H. (1958) Journal of the Physical Soceity of Japan 13:838
Wagner C. (1952) Journal of the Electrochemicals Society 99:369
Wagner C. (1965) Corrosion Science 5:751
Stott F. H., Wood G. C., Stringer J. (1995) Oxidation of Metals 44:113
Zhang G. Z., Gesmundo F., Hou P. H., Niu Y. (2006) Corrosion Science 48:741
Levin E. M., Robbins C. R., McMurdie H. F. (eds) (1964) Phase Diagrams for Ceramists. The American Ceramic Society, Columbus, Ohio
Niu Y., Gesmundo F. (2001) Oxidation of Metals 56:517
Wagner C. (1969) Corrosion Science 9:91
Bastow B. D., Whittle D. P., Wood G. C. (1977) Proceeding of the Royal of London Series A 356:177
Wagner C. (1959) Journal of Electrochemical Soceity 103:627
Acknowledgements
A financial support by the National Natural Scientific Foundation of China (NSFC) under the grants (Nos. 50271079 and 50571107) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niu, Y., Wang, S. & Gesmundo, F. High-Temperature Scaling of Cu–Al and Cu–Cr–Al Alloys: An Example of a Non-Classical Third-Element Effect. Oxid Met 65, 285–306 (2006). https://doi.org/10.1007/s11085-006-9012-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-006-9012-3