Abstract
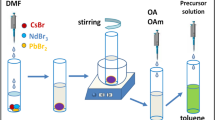
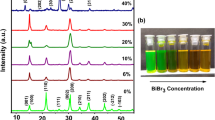
Lead-free bismuth-based perovskite halides are promising materials for optoelectronic applications owing to their lower toxicity, high absorption coefficient, and similar isoelectronic structure to (lead) Pb. Herein, we report the synthesis of Cs3Bi2Br9 perovskite quantum dots (QD) via a modified LARP method and have studied the structural and optical properties. In this study, the QD exhibits an interesting dual emission peak and a bright, unique cyan-green luminescence, with an improved photoluminescence quantum yield of 34%. Studies on Cs3Bi2Br9 QD have been reported earlier, but interestingly the origin of optical properties remains disputed and varies significantly from literature to literature. As a result of our analysis, we expect observed photoluminescence to arise from interconfigurational electronic transitions of Bi3+ ions, with an intense peak at 476 nm attributed to A-band emission and a broad emission at 498 nm attributed to emission from a lower energy level in bismuth ion. Hence, these QDs are suitable for various optoelectronic applications, such as light-emitting diodes (LEDs).





Similar content being viewed by others
Data availability
Available on request to the corresponding author.
References
Akkerman, Q.A., Manna, L.: What defines a halide perovskite? ACS Energy Lett. 5(2), 604–610 (2020)
Awater, R.H., Dorenbos, P.: The Bi3+ 6s and 6p electron binding energies in relation to the chemical environment of inorganic compounds. J. Lumin. 184, 221–231 (2017)
Bass, K.K., Estergreen, L., Savory, C.N., Buckeridge, J., Scanlon, D.O., Djurovich, P.I., Bradforth, S.E., Thompson, M.E., Melot, B.C.: Vibronic structure in room temperature photoluminescence of the halide perovskite Cs3Bi2Br9. Inorg. Chem. 56(1), 42–45 (2017)
Blasse, G., Bril, A.: Investigations on Bi3+-activated phosphors. J. Chem. Phys. 48(1), 217–222 (1968)
Cao, Y., Zhang, Z., Li, L., Zhang, J.R., Zhu, J.J.: An improved strategy for high-quality cesium bismuth bromine perovskite H with remarkable electrochemiluminescence activities. Anal. Chem. 91(13), 8607–8614 (2019)
Chiara, R., Morana, M., Malavasi, L.: Germanium-based halide perovskites: materials, properties, and applications. ChemPlusChem 86(6), 879–888 (2021)
Creutz, S.E., Liu, H., Kaiser, M.E., Li, X., Gamelin, D.R.: Structural diversity in cesium bismuth halide nanocrystals. Chem. Mater. 31(13), 4685–4697 (2019)
Dang, P., Liu, D., Li, G., Al Kheraif, A.A., Lin, J.: Recent advances in bismuth ion-doped phosphor materials: structure design, tunable photoluminescence properties, and application in white LEDs. Adv. Opt. Mater. 8(16), 1901993–1902025 (2020)
Dorenbos, P.: Electronic structure of Bi-activated luminescent compounds and pure bismuth photocatalytic compounds. ECS J Solid State Sci Technol 10(8), 086002–086014 (2021)
Gao, Y., Wu, Y., Lu, H., Chen, C., Liu, Y., Bai, X., Yang, L., William, W.Y., Dai, Q., Zhang, Y.: CsPbBr 3 perovskite nanoparticles as additive for environmentally stable perovskite solar cells with 20.46% efficiency. Nano Energy 59, 517–526 (2019a)
Gao, M., Zhang, C., Lian, L., Guo, J., Xia, Y., Pan, F., Su, X., Zhang, J., Li, H., Zhang, D.: Controlled synthesis and photostability of blue emitting Cs3Bi2Br9 perovskite nanocrystals by employing weak polar solvents at room temperature. J. Mater. Chem. C 7(12), 3688–3695 (2019b)
Ke, W., Kanatzidis, M.G.: Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 10(1), 965–968 (2019)
Kim, J., Park, J., Nam, S.W., Shin, M., Jun, S., Cho, Y.H., Shin, B.: Determining the chemical origin of the photoluminescence of cesium–bismuth–bromide perovskite nanocrystals and improving the luminescence via metal chloride additives. ACS Appl. Energy Mater 3(5), 4650–4657 (2020)
Kojima, A., Teshima, K., Shirai, Y., Miyasaka, T.: Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131(17), 6050–6051 (2009)
Lee, D., Kim, M., Woo, H.Y., Chae, J., Lee, D., Jeon, S., Oh, S.J., Paik, T.: Heating-up synthesis of cesium bismuth bromide perovskite nanocrystals with tailored composition, morphology, and optical properties. RSC Adv. 10(12), 7126–7133 (2020)
Leng, M., Yang, Y., Zeng, K., Chen, Z., Tan, Z., Li, S., Li, J., Xu, B., Li, D., Hautzinger, M.P., Fu, Y.: All-inorganic bismuth-based perovskite quantum dots with bright blue photoluminescence and excellent stability. Adv. Funct. Mater. 28(1), 1704446–1704456 (2018)
Li, X., Gao, X., Zhang, X., Shen, X., Lu, M., Wu, J., Shi, Z., Colvin, V.L., Hu, J., Bai, X., Yu, W.W.: Lead-free halide perovskites for light emission: recent advances and perspectives. Adv. Sci. 8(4), 2003334–2003366 (2021)
Lian, L., Zhai, G., Cheng, F., Xia, Y., Zheng, M., Ke, J., Gao, M., Liu, H., Zhang, D., Li, L., Gao, J.: Colloidal synthesis of lead-free all-inorganic cesium bismuth bromide perovskite nanoplatelets. Cryst. Eng. Comm. 20(46), 7473–7478 (2018)
Liang, L., Gao, P.: Lead-free hybrid perovskite absorbers for viable application: can we eat the cake and have it too? Adv. Sci. 5(2), 1700331–1700363 (2018)
Liang, S., Zhang, M., Biesold, G.M., Choi, W., He, Y., Li, Z., Lin, Z.: Recent advances in synthesis, properties, and applications of metal halide perovskite nanocrystals/polymer nanocomposites. Adv. Mater. 33(50), 2005888–2005923 (2021)
Liu, J., Chen, K., Khan, S.A., Shabbir, B., Zhang, Y., Khan, Q., Bao, Q.: Synthesis and optical applications of low dimensional metal-halide perovskites. Nanotechnology 31(15), 152002 (2020)
Lou, Y., Fang, M., Chen, J., Zhao, Y.: Formation of highly luminescent cesium bismuth halide perovskite quantum dots tuned by anion exchange. Chem Comm 54(30), 3779–3782 (2018)
Nelson, R.D., Santra, K., Wang, Y., Hadi, A., Petrich, J.W., Panthani, M.G.: Synthesis and optical properties of ordered-vacancy perovskite cesium bismuth halide nanocrystals. Chemcomm 54(29), 3640–3643 (2018)
Nie, W., Tsai, H.: Perovskite nanocrystals stabilized in metal–organic frameworks for light emission devices. J. Mater. Chem. A 10(37), 19518–19533 (2022)
Pan, H., Xu, X., Liu, J., Li, X., Zhang, H., Huang, A., Xiao, Z.: Microwave-assisted synthesis of blue-emitting cesium bismuth bromine perovskite nanocrystals without polar solvent. J. Alloys Compd. 886, 161248–161255 (2021)
Ramolahloane, H.T., Nair, G.B., Swart, H.C.: Controlled synthesis and photoluminescence study of blue-emitting Cs3Bi2Br9 nanocrystals prepared by the ligand-assisted reprecipitation (LARP) method. Mater. Res. Bull. 165, 112285–112294 (2023)
Rieger, S., Bohn, B.J., Döblinger, M., Richter, A.F., Tong, Y., Wang, K., Müller-Buschbaum, P., Polavarapu, L., Leppert, L., Stolarczyk, J.K., Feldmann, J.: Excitons and narrow bands determine the optical properties of cesium bismuth halides. Phys. Rev. B 100(20), 201404–201413 (2019)
Samiei, S., Soheyli, E., Vighnesh, K., Nabiyouni, G., Rogach, A.L.: Exploring CsPbX3 (X = Cl, Br, I) perovskite nanocrystals in amorphous oxide glasses: innovations in fabrication and applications. Small 20(17), 2307972-2308004 (2023)
Tan, Z., Li, J., Zhang, C., Li, Z., Hu, Q., Xiao, Z., Kamiya, T., Hosono, H., Niu, G., Lifshitz, E., Cheng, Y.: Highly efficient blue-emitting Bi-doped Cs2SnCl6 perovskite variant: photoluminescence induced by impurity doping. Adv. Func. Mater. 28(29), 1801131–1801140 (2018)
Tran, M.N., Cleveland, I.J., Aydil, E.S.: Resolving the discrepancies in the reported optical absorption of low-dimensional non-toxic perovskites, Cs3Bi2Br9 and Cs3BiBr6. J. Mater. Chem. C 8(30), 10456–10463 (2020)
Wang, Y., Ding, J., Wang, Y.: Preparation and photoluminescence properties with the site-selected excitations of Bi3+-activated Ba3Sc4O9 phosphors. J. Am. Ceram. Soc. 100(6), 2612–2620 (2017)
Wei, Y., Wang, W., Wang, Z., Yang, H., You, X., Zhao, Y., Dang, P., Lian, H., Hao, J., Li, G., Lin, J.: Recent progress of bismuth effect on all-inorganic lead-free metal halide derivatives: crystals structure, luminescence properties, and applications. Adv. Func. Mater. 33(2), 2205829–2205851 (2023)
Wu, H., Liu, W., Ma, W., Liang, T., Liu, X., Fan, J.: Special roles of two-dimensional octahedral frameworks in photodynamics of Cs3Bi2Br 9 nanoplatelets: Electron and lattice-wave localization. Appl. Phys. Lett. 121(18), 181902–181909 (2022)
Yang, B., Chen, J., Hong, F., Mao, X., Zheng, K., Yang, S., Han, K.: Lead-free, air-stable all-inorganic cesium bismuth halide perovskite nanocrystals. Angew. Chem. Int. Ed. 56(41), 12471–12475 (2017)
Zhang, Y., Yin, J., Parida, M.R., Ahmed, G.H., Pan, J., Bakr, O.M., Brédas, J.L., Mohammed, O.F.: Direct-indirect nature of the bandgap in lead-free perovskite nanocrystals. J. Phys. Chem. Lett. 8(14), 3173–3177 (2017)
Acknowledgements
One of the authors Aswathi K.V would like to acknowledge financial support received from Anna University as Anna centenary Research fellowship (ACRF) CFR/ACRF/20257991134/AR1, Dated: 02-01-2020. Authors would like to thank HORIBA- IISc Technical Centre, Bangalore, Dr. Sudeeksha HC for helping with PL lifetime measurements.
Funding
The Funding was provided by Anna University, CFR/ACRF/20257991134/AR1, Dated: 02-01-2020
Author information
Authors and Affiliations
Contributions
Aswathi K V: Conceptualization, Methodology, Investigation, Visualization, Writing–Original Draft. Pandiyarajan: Methodology, Investigation, Visualization Shanthi Subashchandran: Supervision, Conceptualization, Methodology, Writing-Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
K. V, A., Subashchandran, S. & Mariyappan, P. Investigation on optical properties of lead-free Cs3Bi2Br9 perovskite derivative quantum dots synthesised via modified LARP method. Opt Quant Electron 56, 1044 (2024). https://doi.org/10.1007/s11082-024-06975-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-024-06975-7