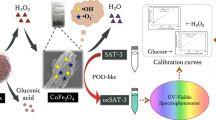
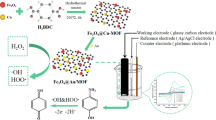
Abstract
Nowadays, environmental contamination is diverse and complicated, and the environment is polluted by multiple harmful chemicals from anthropogenic sources, necessitating the development of efficient and low-cost technologies for monitoring them. In this paper, the hydrothermal technique has been used to synthesize naked Fe3O4, PEG-coated Fe3O4, and CTAB-coated Fe3O4 magnetic nanoparticles (MNPs). X-ray diffraction studies showed that the crystallite sizes of the naked Fe3O4, PEG-coated Fe3O4, and CTAB-coated Fe3O4 NPs were 47.23, 39.23, and 31.70 nm, respectively. All the products had a spherical shape with a hollow structure, according to the FESEM analysis. The VSM revealed that the saturation magnetization of the samples ranged from 82.7 to 77.5 emu/g. By employing unmodified and modified magnetite nanozyme, we present a simple and high-performance sensor for colorimetric detection of phenol. The samples showed different catalytic activity in enhancing the reaction of 4-aminoantipyrine, phenolic species, and H2O2, resulting in a spectacular color shift to signal the target level. This ability of the products was used for the detection of phenol in concentrations ranging from 0.25 μM to 1.2 mM.















Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ahmed, S.R., Cirone, J., Chen, A.: Fluorescent Fe3O4 quantum dots for H2O2 detection. ACS Appl. Nano Mater. 2, 2076–2085 (2019)
Alam, M.N., Chatterjee, A., Das, S., Batuta, S., Mandal, D., Begum, N.A.: Burmese grape fruit juice can trigger the “logic gate”-like colorimetric sensing behavior of Ag nanoparticles towards toxic metal ions. RSC Adv. 5, 23419–23430 (2015)
AL-Jawad, S.M.H., Elttayf, A.K., Saber, A.S.: studying structural, optical, electrical, and sensing properties of nanocrystalline SnO2: Cu films prepared by sol–gel method for co gas sensor application at low temperature. Surf. Rev. Lett. 24, 1750110–1750112 (2017)
AL-Jawad, S.M.H., Imran, N.J., Aboud, K.H.: Synthesis and characterization of Mn:CdS nanoflower thin films prepared by hydrothermal method for photocatalytic activity. J Sol-Gel Sci Technol 100, 423–439 (2021)
Al-Jawad, S.M.H., Salman, O.N., Yousif, N.A.: Influence of titanium tetrachloride concentration and multiple growth cycles of TiO2 nanorod on photoanode performance in dye sensitized solar cell. Photonics Nanostruct. Fundam. Appl. 31, 81–88 (2018)
Alkasir, R.S.J., Ornatska, M., Andreescu, S.: Colorimetric paper bioassay for the detection of phenolic compounds. Anal. Chem. 84, 9729–9737 (2012)
Anambiga, I.V., Suganthan, V., Raj, N.A.N., Buvaneswari, G., Kumar, T.S.S.: Colorimetric detection of lead ions using glutathione stabilized silver nanoparticles. Int. J. Sci. Eng. Res 4, 2229–5518 (2013)
Anbarasu, M., Anandan, M., Chinnasamy, E., Gopinath, V., Balamurugan, K.: Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc 135, 536–539 (2015)
Antarnusa, G., Suharyadi, E.: A synthesis of polyethylene glycol (PEG)-coated magnetite Fe3O4 nanoparticles and their characteristics for enhancement of biosensor. Mater. Res. Express 7, 056103–056106 (2020)
Apostică, A.G., Ichim, T., Radu, V.M., Bulgariu, L.: simple and rapid spectrophotometric method for phenol determination in aqueous media. Bul. Inst. Polit. Iaşi 64, 8–18 (2018)
Arfin, T., Sonawane, K., Tarannum, A.: Review on detection of phenol in water. Adv. Mater. Lett. 10, 753–785 (2019)
Bui, T.Q., Ton, S.N.C., Duong, A.T., Tran, H.T.: Size-dependent magnetic responsiveness of magnetite nanoparticles synthesised by co-precipitation and solvothermal methods. J. Sci.: Adv. Mater. Devices 3, 107–112 (2018)
Çayan, F., Deveci, E., Çayan, G.T., Duru, M.E.: Identifcation and quantifcation of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J. Food Measure. Charact. 14, 1690–1698 (2020)
Çevik, E., Şenel, M., Baykal, A., Abasıyanık, M.: A novel amperometric phenol biosensor based on immobilized HRP on (glycidylmethacrylate)-grafted iron oxide nanoparticles for the determination of phenol derivatives. Sens. Actuat. b: Chem. 173, 396–405 (2012)
Chandane, P., Ladke, J., Jori, C., Deshmukh, S., Zinjarde, S., Chakankar, M., Hocheng, H., Jadhav, U.: Synthesis of magnetic Fe3O4 nanoparticles from scrap iron and use of their peroxidase like activity for phenol detection. J. Environ. Chem. Eng. 7, 103083–103128 (2019)
Cheng, W., Tang, K., Qi, Y., Sheng, J., Liu, Z.: One-step synthesis of superparamagnetic monodisperse porous Fe3O4 hollow and core-shell spheres. J. Mater. Chem. 20, 1799–1805 (2010)
Cheng, Y., Shen, P., Li, X., Li, X., Chu, K., Guo, Y.: Synergistically enhanced peroxidase-like activity of Fe3O4/Ti3C2 MXene quantum dots and its application in colorimetric determination of Cr (VI). Sens. Actuators, B Chem. 376, 132979–132979 (2023)
Chouchaine, A., Kouass, S., Touati, F., Amdouni, N., Dhaouadi, H.: Fe3O4 nanomaterials: synthesis, optical and electrochemical properties. J. Aust. Ceram. Soc. 57, 469–477 (2021)
Fan, K., Cao, C., Yongxin Pan, DLu., Yang, D., Feng, J., Song, L., Liang, M., Yan, Xiyun: Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 7, 459–464 (2012)
Fisli A, Winatapura DS (2018) The surface functionalization of Fe3O4 nanoparticles by CTAB as adsorbent for methyl orange elimination in water. In: IOP Conf. Series: J. Physics: Conf. Series 109, 1012002-012009
Gao, L., Zhuang, J., Nie, L., Zhang, J., Zhang, Y., Gu, N., Wang, T.A., Feng, J., Yang, D., Perrett, S., Yan, X.: Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2, 577–583 (2007)
Gómez, N., Nava, O., Figueroa, L.A., Contreras, R.G., Barrera, A.B., Nestor, A.R.V.: Shape tuning of magnetite nanoparticles obtained by hydrothermal synthesis: effect of temperature. J. Nanomater. 2019, 15 (2019)
Judran, H.K., Yousif, N.A., AL-Jawad, S.M.H.: Preparation and characterization of CdS prepared by hydrothermal method. J Sol-Gel Sci Technol 97, 48–62 (2021)
Kalili, K.M., A.´ Villiers,: Recent developments in the HPLC separation of phenolic compounds. J. Sep. Sci. 34, 854–876 (2011)
Kamakshi, T., Sundari, G.S., Erothu, H., Singh, R.S.: Effect of nickel dopant on structural, morphological and optical characteristics of Fe3O4 nanoparticles. Rasayan J. Chem. 12, 531–536 (2019)
Kaur, M., Mehta, S.K., Kansal, S.K.: Nitrogen doped graphene quantum dots: Efficient fluorescent chemosensor for the selective and sensitive detection of 2,4,6-trinitrophenol. Sens. Actuators, B Chem. 245, 938–945 (2017)
Kumar, P., Khanduri, H., Pathak, S., Singh, A., Basheed, G.A., Pant, R.P.: Temperature selectivity for single phase hydrothermal synthesis of PEG-400 coated magnetite nanoparticles. Transactions 49, 8672–8683 (2020)
Maleh, H.K., Moazampour, M., Ensafi, A.A., Mallakpour, S., Hatami, M.: An electrochemical nanocomposite modified carbon paste electrode as a sensor for simultaneous determination of hydrazine and phenol in water and wastewater samples. Environ. Sci. Pollut. Res. 21, 5879–5888 (2014)
Márquez, F., Herrera, G.M., Campo, T., Cotto, M., Ducongé, J., Sanz, J.M., Elizalde, E., Perales, Ó.R., Morant, C.: Preparation of hollow magnetite microspheres and their applications as drugs carriers. Nanoscale Res. Lett. 7, 210–211 (2012)
Moslemzadeh, M., Larki, A., Ghanemi, K.: A combination of dispersive liquid–liquid microextraction and smartphone-based colorimetric system for the phenol measurement. Microchem. J. 159, 105583 (2020)
Muhsen, M.M., Al-Jawad, S.M.H., Taha, A.A.: Gum Arabic-modifed Mn-doped CuS nanoprisms for cancer photothermal treatment. Chem. Pap. 76, 6821–6838 (2022)
Natalio, F., Andre, R., Hartog, A.F., Stoll, B., Jochum, K.P., Wever, R., Tremel, W.: Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 7, 530–535 (2012)
Rajan, A., Szczepanskac, B.K., Sahu, N.K.: Magneto-thermal response of Fe3O4@CTAB nanoparticles for cancer hyperthermia applications. Mater.Today Commun. 28, 102583–102610 (2021)
Rehani, B.R., Joshi, P.B., Lad, K.N., Pratap, A.: crystallite size estimation of elemental and composite silver nano-powders using XRD principles. Indian J. Pure Appl. Phys. 44, 157–161 (2006)
Rezaei, N., Ehsani, M.H., Aghazadeh, M., Karimzadeh, I.: An investigation on magnetic-interacting Fe3O4 nanoparticles prepared by electrochemical synthesis method. J. Supercond. Novel Magn. 31, 2139–2147 (2018)
Sergeyeva, T.A., Slinchenko, O.A., Gorbach, L.A., Matyushov, V.F., Brovko, O.O., Piletskyc, S.A., Sergeev, L.M., Elska, G.V.: Catalytic molecularly imprinted polymer membranes: development of the biomimetic sensor for phenols detection. Anal. Chim. Acta 659, 274–279 (2010)
Shi, W., Wang, Q., Long, Y., Cheng, Z., Chen, S., Zheng, H., Huang, Y.: Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 47, 6695–6697 (2011)
Sindhu, R.K., Najda, A., Kaur, P., Shah, M., Singh, H., Kaur, P., Cavalu, S., Sierocinska, M.J., Rahman, Md.H.: Potentiality of nanoenzymes for cancer treatment and other diseases: current status and future challenges. Materials 14, 5965 (2021)
Stanek, N., Kafarski, P., Misiak, I.J.: Development of a high performance thin layer chromatography method for the rapid qualification and quantification of phenolic compounds and abscisic acid in honeys. J. Chromatogr. A 1598, 209–215 (2019)
Szabo, R., Gaspar, A.: Determination of phenolic compounds by capillary zone electrophoresis-mass spectrometry. Molecules 27, 4540–4549 (2022)
Taha, A.A., AL-Jawad, S.M.H., Salim, M.M.: Influence of titanium tetraisopropoxide concentration on the antibacterial activity of TiO2 thin films. Surf. Rev. Lett. 25, 1850111–185118 (2018)
Tan, W., Xin, R., Zhang, J., Yang, L., Jing, M., Ma, F., Yang, J.: Article Co(II)-based metal-organic framework derived CA-CoNiMn-CLDHs with peroxidase-like activity for colorimetric detection of phenol. Materials 16, 6212 (2023)
Terohid, S.A.A., Heidari, S., Jafari, A., Asgary, S.: Effect of growth time on structural, morphological and electrical properties of tungsten oxide nanowire. Appl. Phys. A 124, 567 (2018)
Wang, X., Huang, H., Li, G., Liu, Y., Huang, J., Yang, D.-P.: Hydrothermal synthesis of 3D hollow porous Fe3O4 microspheres towards catalytic removal of organic pollutants. Nanoscale Res. Lett. 9, 9648-9650 (2014)
Wei, H., Wang, E.: Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 80, 2250–2254 (2008)
Wua, S., Guo, D., Xua, X., Pan, J., Niu, X.: Colorimetric quantification and discrimination of phenolic pollutants based on peroxidase-like Fe3O4 nanoparticles. Sens. Actuators, B Chem. 303, 127225–127228 (2020)
Yana, H., Liping, Z., Weiwei, H., Xiaojuan, L., Xiangnong, L., Yuxiang, Y.: A study on synthesis and properties of Fe3O4 nanoparticles by solvothermal method. Glass Phys. Chem 36, 325–331 (2010)
Yanga, J., Zoua, P., Yanga, L., Cao, J., Sun, Y., Han, D., Yang, S., Wanga, Z., Chena, G., Wang, B., Konga, X.: A comprehensive study on the synthesis and paramagnetic properties of PEG-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 303, 425–432 (2014)
Ye, M., Zhu, Y., Lu, Y., Gan, L., Zhang, Y., Zhao, Y.: Magnetic nanomaterials with unique nanozymes-like characteristics for colorimetric sensors: a review. Talanta 230, 122299–122322 (2021)
Yousif, N.A., Al-Jawad, S.M.H.: Influence of Laser wavelength on morphological and optical properties of ZnO nanoparticles prepared by laser ablation in water. J. Phys: Conf. Ser. 1795, 012056–012059 (2021)
Zhang, J., Zhuang, J., Gao, L., Zhang, Y., Gu, N., Feng, J., Yang, D., Zhu, J., Yan, X.: Decomposing phenol by the hidden talent of ferromagnetic nanoparticles. Chemosphere 73, 1524–1528 (2008)
Zhang, S., Zhao, X., Niu, H., Shi, Y., Cai, Y., Jiang, G.: Superparamagnetic Fe3O4 nanoparticles as catalysts for the catalytic oxidation of phenolic and aniline compounds. J. Hazard. Mater. 167, 560–566 (2009)
Zhang, W., Shen, F., Hong, R.: Solvothermal synthesis of magnetic Fe3O4 microparticles via self-assembly of Fe3O4 nanoparticles. Particuology 9, 179–186 (2011)
Zhong, N., Chen, M., Wang, Z., Xin, X., Li, B.: Photochemical device for selective detection of phenol in aqueous solutions. Lab Chip 18, 1621–1632 (2018)
Acknowledgements
We would like to thank the University of Technology and the School of Applied Sciences in Baghdad, Iraq, for conducting this study.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
NAY contributed to investigation, writing—original draft, methodology, and formal analysis. SMH. AJ contributed to writing—review and editing, administration, formal analysis, and investigation. AAT contributed to writing formal analysis, and investigation.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Ethical approval
None required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yousif, N.A., AL-Jawad, S.M.H. & Taha, A.A. Synthesis and characterization of uncoated and coated magnetite nanoparticles and use of their peroxidase like activity for phenol detection. Opt Quant Electron 55, 1294 (2023). https://doi.org/10.1007/s11082-023-05605-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-05605-y