Abstract
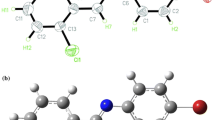
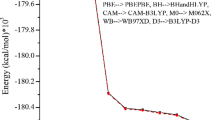
The enhancement of Nonlinear optical (NLO) activity of the 2-nitrotoulene (2NT) after the substitution of the halogens (F, Cl, Br, and I) at the para position of the benzene ring was reported in the paper. All the computational details were mentioned using density functional theory with B3LYP/6-311++G(d,p) basis set. The engagement of the electrophilic and nucleophilic regions in molecules was established using contour plots. Mulliken charge analysis and frontier molecular orbital parameters laid the high chemical reactivity of 2NT molecule after the substitution of F, Cl, Br, and I. Using time-dependent density functional theory, electronic properties were analyzed by computing absorption and emission spectra. The higher Raman intensity modes and higher absorbance intensity curve for iodine-substituted 2NT (2NT-I) highlighted the highest reactivity. The lowest band gap was reported for 2NT-I (4.02 eV) which better correlates with the charge and spectral findings. The polarizability parameters set a fair comparison between the NLO activities of the molecules. 2NT-I has the highest values of polarizability parameters among the other molecules.







Similar content being viewed by others
Availability of data and materials
All data generated or analysed during this study, which support the plots within this paper and the other findings of this study, are included in this article and it is Supplementary Information. Source data are provided with this paper.
References
Badran, H.A., Al-Maliki, A., Alfahed, R.K.F., et al.: Synthesis, surface profile, nonlinear reflective index and photophysical properties of curcumin compound. J. Mater. Sci. Mater. Electron. 29, 10890–10903 (2018). https://doi.org/10.1007/s10854-018-9167-0
Bhatt, T., Pant, T., Dhondiyal, C.C., Rana, M., Chowdhury, P., Joshi, G.C., Arya, G.C., Tiwari, H.: Computational study of the intermolecular interactions and their effect on the UV–visible spectra of the ternary liquid mixture of benzene, ethanol and propylene glycol. J Mol. Model. 26, 268 (2020). https://doi.org/10.1007/s00894-020-04533-y
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648 (1993). https://doi.org/10.1063/1.464913
Becke, A.D.: Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals. J. Chem. Phys. 107, 8554–8560 (1997). https://doi.org/10.1063/1.475007
Boateng, D.A., Kayla, M., Word, D., Gutsev, L.G., Jena, P., Tibbetts, K.M.: Conserved vibrational coherence in the ultrafast rearrangement of 2-nitrotoluene radical cation. J. Phys. Chem. A 123(6), 1140–1152 (2019). https://doi.org/10.1021/acs.jpca.8b11723
Cassidy, C., Halbout, J.M., Donaldson, W., Tang, C.L.: Nonlinear optical properties of urea. Opt. Commun. 29(2), 243–246 (1979). https://doi.org/10.1016/0030-4018(79)90027-0
Dennington, R., Keith, T., Millam, J.: GaussView, Version 4.1.2, Semichem, Inc., Shawnee Mission, KS (2007).
Ejuh, G.W., Nya, F.T., Djongyang, N., et al.: Study of some properties of quinone derivatives from quantum chemical calculations. Opt. Quantum Electron. 50, 336 (2018). https://doi.org/10.1007/s11082-018-1603-0
Eşme, A., Sagdinc, S.G.: Conformational, spectroscopic (FT-IR, FT-Raman, and UV–Vis), and molecular docking studies of N-(2-hydroxyethyl) succinimide. J. Mol. Struct. 1195, 451–461 (2019). https://doi.org/10.1016/j.molstruc.2019.06.019
Felscia, U.R., Rajkumar, B.J.M.: Computational study of quinacridone on silver and gold clusters: applications to organic light emitting diodes and nonlinear optical devices. Mater. Lett. (2018). https://doi.org/10.1016/j.matlet.2018.03.149
Felscia, U.R., Rajkumar, B.J.M., Sankar, P., Philip, R., Mary, M.B.: Theoretical and experimental investigations of nitropyrene on silver for nonlinear optical and metal ion sensing applications. Mater. Chem. Phys. (2019). https://doi.org/10.1016/j.matchemphys.2019.122466
Frisch, M.J.: Gaussian 09, Revision B.01, Gaussian Inc., Wallingford CT (2010).
Jessen, N.I., Bertuzzi, G., Bura, M., Skipper, M.L., Jørgensen, K.L.: Enantioselective construction of the Cycl[3.2.2]azine core via organocatalytic [12 + 2] cycloadditions. J. Am. Chem. Soc (2021). https://doi.org/10.1021/jacs.1c00499
Jeyaram, S.: Spectral, third-order nonlinear optical and optical switching behavior of β-carotenoid extracted from phyllanthus niruri. Indian J Phys. 96, 1655–1661 (2022). https://doi.org/10.1007/s12648-021-02122-0
Jibin, S., Yantao, S., Chaoxian, Y., Dongxu, L., Zheng, X., Shuyun, Z., Chengshan, Y., Li, Z.H., Xiangfeng, S.: Remarkable nonlinear optical response of pyrazine-fused trichalcogenasumanenes and their application for optical power limiting. J. Mol. Struct. 6(48), 13114–13119 (2018). https://doi.org/10.1039/c8tc04778b
John, N.L., Abraham, S., Sajan, D., Sarojini, B.K., Narayana, B.: Quantum chemical studies of molecular structure, vibrational spectra and nonlinear optical properties of p-iodoaniline and p-bromoaniline. J. Mol. Struct. 1222, 128939 (2020). https://doi.org/10.1016/j.molstruc.2020.128939
Khan, M.U., Khalid, M., Khera, R.A., Akhtar, M.N., Abbas, A., Rehman, M.F., Braga, A.A.C., Alam, M.M., Imran, M., Wang, Y., Lu, C.: Influence of acceptor tethering on the performance of nonlinear optical properties for pyrene-based materials with A-π-D-π-D architecture. Arab. J. Chem. 15(3), 103673 (2022). https://doi.org/10.1016/j.arabjc.2021.103673
Koopmans, T.: Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1, 104–113 (1933). https://doi.org/10.1016/S0031-8914(34)90011-2
Krishnakumar, V., Murugeswari, K., Prabavathi, N., Mathammal, R.: Molecular structure, vibrational spectra, HOMO, LUMO and NMR studies of 2-chloro-4-nitrotoluene and 4-chloro-2-nitrotoluene. Spectrochim. Acta A Mol. 91, 1–10 (2012). https://doi.org/10.1016/j.saa.2012.01.038
Lakhera, S., Devlal, K., Ghosh, A., Rana, M.: In silico investigation of phytoconstituents of medicinal herb ‘Piper Longum’ against SARS-CoV-2 by molecular docking and molecular dynamics analysis. Results Chem. 3, 100199 (2021). https://doi.org/10.1016/j.rechem.2021.100199
Lakhera, S., Rana, M., Devlal, K.: Theoretical study on spectral and optical properties of essential amino acids: a comparative study. Opt. Quantum Electron. 54, 714 (2022a). https://doi.org/10.1007/s11082-022-04118-4
Lakhera, S., Devlal, K., Rana, M., Celik, I.: Study of nonlinear optical responses of phytochemicals of Clitoria ternatea by quantum mechanical approach and investigation of their anti-Alzheimer activity with in silico approach. Struct. Chem. (2022b). https://doi.org/10.1007/s11224-022-01981-5
Lakhera, S., Devlal, K., Rana, M., Dhuliya, V.: Quantum mechanical study of three aromatic bioactive fatty alcohol compounds with nonlinear optical and potential light harvesting properties. Opt. Mater. 129, 112476 (2022c). https://doi.org/10.1016/j.optmat.2022.112476
Lakhera, S., Rana, M., Devlal, K.: Modelling the reactivity of entrectinib and evaluation of its potential anticancer activity using molecular docking approach. DAE Solid State Phys. Symp 55, 583–584 (2022d)
Lakhera, S., Devlal, K., Ghosh, A., Rana, M.: Modelling the DFT structural and reactivity study of feverfew and evaluation of its potential antiviral activity against COVID-19 using molecular docking and MD simulations. Chem. Pap. 76, 2759–2776 (2022e). https://doi.org/10.1007/s11696-022-02067-6
Lakhera, S., Rana, M., Devlal, K., Celik, I., Yadav, R.: A comprehensive exploration of pharmacological properties, bioactivities and inhibitory potentiality of luteolin from Tridax procumbens as anticancer drug by in-silico approach. Struct. Chem. (2022f). https://doi.org/10.1007/s11224-022-01882-7
Lakhera, S., Rana, M., Devlal, K.: Investigation of nonlinear optical response of organic compound pyrrolidine-2,5-dione. DAE Solid State Phys. Symp. 55, 585–586 (2022g)
Lakhera, S., Rana, M., Devlal, K.: Infuence of adsorption of gold and silver nanoclusters on structural, electronic, and nonlinear optical properties of pentacene‑5,12‑dione: a DFT study. Opt. Quant. Electr. 55,178 (2023). https://doi.org/10.1007/s11082-022-04422-z
Lin, M.F., Lee, Y.E., Ni, C.K., Xu, S., Lin, M.C.: Photodissociation dynamics of nitrobenzene and o-nitrotoluene. Chem. Phys. 126(6), 064310 (2007). https://doi.org/10.1063/1.2435351
Liu, Y., Wang, R., Wang, Z., et al.: Formation of twelve-fold iodine coordination at high pressure. Nat. Commun. 13, 412 (2022). https://doi.org/10.1038/s41467-022-28083-4
Maharramov, A.M., Shikhaliyev, N.Q., Suleymanova, G.T., Gurbanov, A.V., Babayeva, G.V., Mammadova, G.Z., Zubkov, F.I., Nenajdenko, V.G., Mahmudov, K.T., Pombeiro, A.J.L.: Pnicogen, halogen and hydrogen bonds in (E)-1-(2,2-dichloro-1-(2-nitrophenyl)vinyl)-2-(para-substituted phenyl)-diazenes. Dyes Pigments 159, 135–141 (2018). https://doi.org/10.1016/j.dyepig.2018.06.022
Majee, P., Singha, D.K., Mondal, S.K., Mahata, P.: Effect of charge transfer and structural rigidity on divergent luminescence response of a metal organic framework towards different metal ions: luminescence lifetime decay experiments and DFT calculations. PPS (2019). https://doi.org/10.1039/C9PP00024K
Mohan, A., Malathi, M.: Dielectric relaxation and thermodynamic studies of binary mixtures of 2-nitrotoluene with primary and secondary alcohols at different temperatures. J. Solut. Chem. 47, 667–683 (2018). https://doi.org/10.1007/s10953-018-0744-x
Morosanu, A., Cezarina, B., Andreea, C., Babusca, D., Dimitriu, D.G., Dorohoi, O.D.: Quantum mechanical and solvatochromic characterization of quercetin. Anal. Lett. 50(17), 2725–2739 (2017). https://doi.org/10.1080/00032719.2017.1291657
Moroz, T.N., Edwards, H.G.M.: The use of Raman and infrared spectroscopy in determining the space symmetry group among the groups with the same rules of systematic absence in the diffraction patterns: some basic principles and applications. J. Raman Spectrosc. 52, 2058–2067 (2021). https://doi.org/10.1002/jrs.6220
Nayak, S., Manjunatha, K.B., Goveas, L.C., et al.: Investigation of nonlinear optical properties of AgNPs synthesized using Cyclea peltata leaf extract post OVAT optimization. BioNanoScience 11, 884–892 (2021). https://doi.org/10.1007/s12668-021-00875-w
Ojo, N.D., Krause, R.W., Obi-Egbedi, N.O.: Electronic and nonlinear optical properties of 3-(((2-substituted-4-nitrophenyl)imino)methyl)phenol. Comput. Theor. Chem. 1192, 1–8 (2020). https://doi.org/10.1103/PhysRevX.11.021067
Pandith, A.H., Islam, N.: Electron transport and nonlinear optical properties of substituted aryldimesityl boranes: a DFT study. PLoS ONE 9(12), e114125 (2014). https://doi.org/10.1371/journal.pone.0114125
Parales, R.E., Huang, R., Yu, C.L., Parales, J.V., Lee, F.K.N., Lessner, D.J., Ivkovic-Jensen, M.M., Liu, W., Friemann, R., Ramaswamy, S., Gibson, D.T.: Purification, Characterization, and crystallization of the components of the nitrobenzene and 2-nitrotoluene dioxygenase enzyme systems. Appl. Environ. Microbiol. 71(7), 3806–3814 (2005). https://doi.org/10.1128/AEM.71.7.3806-3814.2005
Ramalingam, A., Kuppusamy, M., Sambandam, S., Medimagh, M., Oyeneyin, O.E., Shanmugasundaram, A., Issaoui, N., Ojo, N.D.: Synthesis, spectroscopic, topological, hirshfeld surface analysis, and anti-covid-19 molecular docking investigation of isopropyl 1-benzoyl-4-(benzoyloxy)-2,6-diphenyl-1,2,5,6-tetrahydropyridine-3-carboxylate. Heliyon 8(10), e10831 (2022). https://doi.org/10.1016/j.heliyon.2022.e10831
Rana, M., Chowdhury, P.: L-glutathione capped CdSeS/ZnS quantum dots as an environmentally hazardous chemical sensor. J. Appl. Eng. 2(16), 1319–1321 (2015)
Rana, M., Devlal, K.: Thioglycolic acid capped CdTe quantum dots as sensors for the detection of hazardous heavy metal ion Cu2+ in water. Mapan 37, 41–46 (2022). https://doi.org/10.1007/s12647-021-00479-5
Rana, M., Singla, N., Chatterjee, A., Shukla, A., Chowdhury, P.: Investigation of nonlinear optical (NLO) properties by charge transfer contributions of amine-functionalized tetraphenylethylene. Opt. Mater. 62, 80–89 (2016). https://doi.org/10.1016/j.optmat.2016.09.043
Rana, M., Singla, N., Pathak, A., Dhanya, R., Narayana, C., Chowdhury, P.: Vibrational-electronic properties of intra/inter molecular hydrogen bonded heterocyclic dimer: an experimental and theoretical study of pyrrole-2-carboxaldehyde. Vib. Spectrosc. 89, 16–25 (2017). https://doi.org/10.1016/j.vibspec.2016.12.003
Rana, M., Banerjee, C., Chowdhury, P.: Studies on optical signal due to oxygen effect on hydrogenated amorphous/crystalline silicon thin films. Appl. Phys. a. 127, 192 (2021). https://doi.org/10.1007/s00339-021-04322-1
Revathi, V., Rajendran, V.: Investigation about nonlinear optics and antibacterial activity of pyrrolidine-2-carboxylic acid cadmium chloride hydrate single crystal. Optik 154, 234–241 (2018). https://doi.org/10.1016/j.ijleo.2017.10.060
Riega, H.D., Gunawidjaja, R., Eilers, H.: Photoluminescence spectroscopy of 2-nitrotoluene and its photo and photothermal decomposition derivatives. J. Photochem. Photobiol. 268, 50–57 (2013). https://doi.org/10.1016/j.jphotochem.2013.06.020
Sakunthaladevi, R., Jothi, L.: Chemical growth dynamics of 4-methyl-4′-hydroxy benzylidene aniline NLO single crystal structure and spectroscopic applications. J. Mol. Struct. 1233, 130054 (2021). https://doi.org/10.1016/j.molstruc.2021.130054
Sangeetha, K., Prasad, L.G., Mathammal, R.: Structural elucidation and physicochemical properties of an organic NLO crystal: 4-nitrotoluene-2-sulphonic acid dihydrate. J. Mol. Struct. 1155, 598–609 (2018). https://doi.org/10.1016/j.molstruc.2017.11.048
Sathyavathi, R., Krishna, K.M., Rao, S.V., Venugopal, S.V., Rao, D.N.: Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Adv. Sci. Lett. 3(2), 138–143 (2010). https://doi.org/10.1166/asl.2010.1099
Sindhusha, S., Padma, C.M., Thayanithi, V.: Experimental and theoretical investigations of organic creatininium 2-chloroacetate nonlinear optical single crystal. J. Mater. Sci. Mater. Electron. 32, 6498–6510 (2021). https://doi.org/10.1007/s10854-021-05367-x
Singla, N., Tripathi, A., Rana, M., Goswami, S.K., Pathak, A., Chowdhury, P.: Turn on/off” proton transfer based fluorescent sensor for selective detection of environmentally hazardous metal ions (Zn2+, Pb2+) in aqueous media. J. Lumin. 165, 46–55 (2015). https://doi.org/10.1016/j.jlumin.2015.04.007
Song, Y.Z., Wang, J., Li, M.T., et al.: Electrochemical catalytic reduction of p-nitrotoluene on the surface of α-Ag2S crystal. Russ. J. Phys. Chem. 96, 899–906 (2022). https://doi.org/10.1134/S003602442204029X
Sukma, H.A., Hendra, H., Steven, K.Y.: Investigation of the chemical and optical properties of halogen-substituted N-methyl-4-piperidone curcumin analogs by density functional theory calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1233, 117152 (2019). https://doi.org/10.1016/j.saa.2019.117152
Weeraratna, C., Amarasinghe, C., Lu, W., Ahmed, M.: A direct probe of the hydrogen bond network in aqueous glycerol aerosols. J. Phys. Chem. Lett. 12(23), 5503–5511 (2021). https://doi.org/10.1021/acs.jpclett.1c01383
Wen, L., Zhou, L., Zhang, B., Bingguang, M., Meng, X., Qu, H., Hua, D., Li, D.: Multifunctional amino-decorated metal–organic frameworks: nonlinear-optic, ferroelectric, fluorescence sensing and photocatalytic properties. J. Mater. Chem. 22(42), 22603 (2012). https://doi.org/10.1039/c2jm34349e
Yadav, P., Rana, M., Chowdhury, P.: DFT and MD simulation investigation of favipiravir as an emerging antiviral option against viral protease (3CLpro) of SARS-CoV-2. J. Mol. Struct. 1246, 131253 (2021). https://doi.org/10.1016/j.molstruc.2021.131253
Yakovenko, A.A., Antipin, M.Y., Timofeeva, T.V.: Molecular and crystal structure of low melting nitrotoluene isomers. Cryst. Growth Des. 9(1), 57–65 (2009). https://doi.org/10.1021/cg800659f
Yanxin, Y., Yulin, H., Pan, L., Hui, Z., Qiuying, Z., Jialiang, L., Linghan, X., Xibin, W., Yuhui, A., Ming, L.: Mild and in situ photo-crosslinking of anthracene-functionalized poly (aryl ether ketone) for enhancing temporal stability of organic NLO materials. J. Mater. Sci. 56, 5910–5923 (2021). https://doi.org/10.1007/s10853-020-05594-3
Yanzhu, L., Qingyan, S., Hongbo, Z., Hongya, G., Dongping, L., Yongxiu, L.: One-dimensional Europium-coordination polymer as luminescent sensor for highly selective and sensitive detection of 2,4,6-trinitrophenol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 264, 120303 (2022). https://doi.org/10.1016/j.saa.2021.120303
Zaini, M.F., Arshad, S., Thanigaimani, K., Khalib, N.C., Zainuri, D.A., Abdullah, M., Razak, I.A.: New halogenated chalcones: synthesis, crystal structure, spectroscopic and theoretical analyses for third-order nonlinear optical properties. J. Mol. Struct. 1195, 606–619 (2019). https://doi.org/10.1016/j.molstruc.2019.05.122
Funding
The authors declare that this research received no specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
SL: Data curation, Writing-Original draft preparation, Visualization, Investigation, Software, Validation. MR: Conceptualization, Methodology, Writing-Reviewing and Editing, Supervision. KD: Conceptualization, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This material is the authors’ own original work, which has not been previously published elsewhere. All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content. The paper properly credits the meaningful contributions of all the co-authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lakhera, S., Rana, M. & Devlal, K. Investigation of the electronic and optical activity of halogen-substituted 2-nitrotoulene by density functional theory. Opt Quant Electron 55, 292 (2023). https://doi.org/10.1007/s11082-023-04569-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-04569-3