Abstract
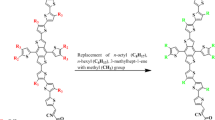
Current research has focused on utilization of non-fullerene based organic materials for the advancement of nonlinear optical (NLO) based technology. The reference compound (DTPSR1) was used in tailoring process to design seven new derivatives (DTPSD2-DTPSD8) via various acceptor moieties. The M06-2X level with 6-311G(d,p) basis set was used for assessing frontier molecular orbitals (FMOs), natural bonding orbital (NBO), nonlinear optical properties [average polarizability < α > , first hyperpolarizability (βtotal), second hyperpolarizability (γtotal)], transition density matrix (TDM) and UV–Vis analyses of DTPSR1 and DTPSD2-DTPSD8. The UV–Vis analysis indicated that the designed derivatives show comparable results (515.462–586.269 nm) with reference molecule (583.592 nm), except DTPSD7, that exhibited slight red shift (586.269 nm). Smaller LUMO–HOMO energy gaps were reported as in DTPSD3 (3.53 eV), DTPSD7 (3.53 eV) and DTPSD8 (3.55 eV) as compared to DTPSR1 (3.60 eV) which was further supported by TDM analysis. The global reactivity descriptors have also shown close correlation with LUMO–HOMO energy gaps; smaller value of energy gap showed lower hardness value 1.77 eV for DTPSD3, DTPSD7 and DTPSD8 and greater softness values 0.283 eV for DTPSD3, DTPSD7 and 0.281 eV for DTPSD8, respectively. The hyper conjugative interactions, stability, and electron-transfer mechanism were elucidated by using NBO analysis. DTPSD2-DTPSD8 also exhibited comparatively closer NLO results with DTPSR1. Among DTPSD2–DTPSD8, the highest ⟨α⟩1439.16 a.u, βtotal 189,720.546 a.u and γtotal 1.980890 × 107 a.u were observed for DTPSD7. It is anticipated that our study would provide a springboard to attain the NLO materials exhibiting significant future applications such as in telecommunication, data storage and optical poling.







Similar content being viewed by others
References
Ahmad, M.S., Khalid, M., Shaheen, M.A., Tahir, M.N., Khan, M.U., Braga, A.A.C., Shad, H.A.: Synthesis and XRD, FT-IR vibrational, UV–vis, and nonlinear optical exploration of novel tetra substituted imidazole derivatives: A synergistic experimental-computational analysis. J. Phys. Chem. Solids 115, 265–276 (2018)
Akram, M., Adeel, M., Khalid, M., Tahir, M.N., Khan, M.U., Asghar, M.A., Ullah, M.A., Iqbal, M.: A combined experimental and computational study of 3-bromo-5-(2, 5-difluorophenyl) pyridine and 3, 5-bis (naphthalen-1-yl) pyridine: Insight into the synthesis, spectroscopic, single crystal XRD, electronic, nonlinear optical and biological properties. J. Mol. Struct. 1160, 129–141 (2018)
Amiri, S.S., Makarem, S., Ahmar, H., Ashenagar, S.: Theoretical studies and spectroscopic characterization of novel 4-methyl-5-((5-phenyl-1, 3, 4-oxadiazol-2-yl) thio) benzene-1, 2-diol. J. Mol. Struct. 1119, 18–24 (2016)
Ans, M., Iqbal, J., Ahmad, Z., Muhammad, S., Hussain, R., Eliasson, B., Ayub, K.: Designing three-dimensional (3D) non-fullerene small molecule acceptors with efficient photovoltaic parameters. ChemistrySelect 3(45), 12797–12804 (2018)
Barone, V., Cossi, M.: Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102(11), 1995–2001 (1998)
Bi, W., Louvain, N., Mercier, N., Luc, J., Rau, I., Kajzar, F., Sahraoui, B.: A switchable NLO organic-inorganic compound based on conformationally chiral disulfide molecules and Bi (III) I5 iodobismuthate networks. Adv. Mater. 20(5), 1013–1017 (2008)
Breitung, E.M., Shu, C.-F., McMahon, R.J.: Thiazole and thiophene analogues of donor− acceptor stilbenes: molecular hyperpolarizabilities and structure− property relationships. J. Am. Chem. Soc. 122(6), 1154–1160 (2000)
Chattaraj, P.K., Roy, D.R.: Update 1 of: electrophilicity index. Chem. Rev. 107(9), PR46–PR74 (2007)
Chattaraj, P., Giri, S., Duley, S.: Update 2 of: electrophilicity index. Chem. Rev. 111(2), PR43–PR75 (2011)
Chemla, D.S.: Nonlinear Optical Properties of Organic Molecules and Crystals V1, vol. 1. Elsevier (2012)
Cheng, P., Li, G., Zhan, X., Yang, Y.: Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 12(3), 131–142 (2018)
Dennington, R., Keith, T.A., Millam, J.M.: GaussView 6.0. 16. Semichem Inc. (2016)
Eaton, D.F.: Nonlinear Optical Materials: The Great and Near Great. ACS Publications (1991)
Fonseca, R.D., Vivas, M.G., Silva, D.L., Eucat, G., Bretonnière, Y., Andraud, C., De Boni, L., Mendonca, C.R.: First-order hyperpolarizability of triphenylamine derivatives containing cyanopyridine: molecular branching effect. J. Phys. Chem. C 122(3), 1770–1778 (2018)
Frisch, M., Clemente, F.: Gaussian 09, revision a. 01, mj frisch, gw trucks, hb schlegel, ge scuseria, ma robb, jr cheeseman, g. Scalmani, V. Barone, B. Mennucci, GA Petersson, H. Nakatsuji, M. Caricato, X. Li, HP Hratchian, AF Izmaylov, J. Bloino, G. Zhe, pp. 20–44 (2009)
Fukui, K.: Role of frontier orbitals in chemical reactions. Science 218(4574), 747–754 (1982)
Glendening, E.D., Weinhold, F.: Natural resonance theory: I. General formalism. J. Comput. Chem. 19(6), 593–609 (1998)
Gunasekaran, S., Balaji, R.A., Kumeresan, S., Anand, G., Srinivasan, S.: Experimental and theoretical investigations of spectroscopic properties of N-acetyl-5-methoxytryptamine. Can. J. Anal. Sci. Spectrosc. 53(4), 149–162 (2008)
Guo, L., Guo, Z., Li, X.: Design and preparation of side chain electro-optic polymeric materials based on novel organic second order nonlinear optical chromophores with double carboxyl groups. J. Mater. Sci. Mater. Electron. 29(3), 2577–2584 (2018)
Halasyamani, P.S., Zhang, W.: Inorganic Materials for UV and Deep-UV Nonlinear-Optical Applications, vol. 56, pp. 12077–12085. ACS Publications (2017)
Hanwell, M.D., Curtis, D.E., Lonie, D.C., Vandermeersch, T., Zurek, E., Hutchison, G.R.: Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics 4(1), 1–17 (2012)
Haroon, M., Mahmood, R., Janjua, M.R.S.A.: An Interesting behavior and nonlinear optical (NLO) response of hexamolybdate metal cluster: theoretical insight into electro-optic modulation of hybrid composites. J. Cluster Sci. 28(5), 2693–2708 (2017)
He, Y., Li, Y.: Fullerene derivative acceptors for high performance polymer solar cells. Phys. Chem. Chem. Phys. 13(6), 1970–1983 (2011)
He, S., Tan, Y., Xiao, X., Zhu, L., Guo, Y., Li, M., Tian, A., Pu, X., Wong, N.-B.: Substituent effects on electronic character of the CN group and trans/cis isomerization in the C-substituted imine derivatives: a computational study. J. Mol. Struct. Thoechem. 951(1–3), 7–13 (2010)
Janjua, M.R.S.A.: Quantum mechanical design of efficient second-order nonlinear optical materials based on heteroaromatic imido-substituted hexamolybdates: first theoretical framework of POM-based heterocyclic aromatic rings. Inorg. Chem. 51(21), 11306–11314 (2012)
Janjua, M.R.S.A., Su, Z.-M., Guan, W., Liu, C.-G., Yan, L.-K., Song, P., Maheen, G.: Tuning second-order non-linear (NLO) optical response of organoimido-substituted hexamolybdates through halogens: Quantum design of novel organic-inorganic hybrid NLO materials. Aust. J. Chem. 63(5), 836–844 (2010)
Janjua, M.R.S.A., Khan, M.U., Bashir, B., Iqbal, M.A., Song, Y., Naqvi, S.A.R., Khan, Z.A.: Effect of π-conjugation spacer (CC) on the first hyperpolarizabilities of polymeric chain containing polyoxometalate cluster as a side-chain pendant: a DFT study. Comput. Theor. Chem. 994, 34–40 (2012b)
Janjua, M.R.S.A., Jamil, S., Ahmad, T., Yang, Z., Mahmood, A., Pan, S.: Quantum chemical perspective of efficient NLO materials based on dipolar trans-tetraammineruthenium (II) complexes with pyridinium and thiocyanate ligands: first theoretical framework. Comput. Theor. Chem. 1033, 6–13 (2014)
Janjua, M.R.S.A., Jamil, S., Mahmood, A., Zafar, A., Haroon, M., Bhatti, H.N.: Solvent-dependent non-linear optical properties of 5, 5′-disubstituted-2, 2′-bipyridine complexes of ruthenium (II): a quantum chemical perspective. Aust. J. Chem. 68(10), 1502–1507 (2015a)
Janjua, M.R.S.A., Yamani, Z.H., Jamil, S., Mahmood, A., Ahmad, I., Haroon, M., Tahir, M.H., Yang, Z., Pan, S.: First principle study of electronic and non-linear optical (NLO) properties of triphenylamine dyes: interactive design computation of new NLO compounds. Aust. J. Chem. 69(4), 467–472 (2015b)
Janjua, M. R. S. A., Amin, M., Ali, M., Bashir, B., Khan, M. U., Iqbal, M. A., Guan, W., Yan, L., Su, Z. M.: A DFT study on the two‐dimensional second‐order nonlinear optical (NLO) response of terpyridine‐substituted hexamolybdates: physical insight on 2D inorganic–organic hybrid functional materials (2012a)
Katono, M., Wielopolski, M., Marszalek, M., Bessho, T., Moser, J.-E., Humphry-Baker, R., Zakeeruddin, S.M., Grätzel, M.: Effect of extended π-conjugation of the donor structure of organic D-A− π–A dyes on the photovoltaic performance of dye-Sensitized solar cells. J. Phys. Chem. C 118(30), 16486–16493 (2014)
Khalid, M., Ali, M., Aslam, M., Sumrra, S.H., Khan, M.U., Raza, N., Kumar, N., Imran, M.: Frontier molecular, Natural bond orbital, UV-Vis spectral stduy, Solvent influence on geometric parameters, Vibrational frequencies and solvation energies of 8-Hydroxyquinoline. Int. J. Pharm. Sci. Res. 8(2), 457–469 (2017)
Khalid, M., Ali, A., Jawaria, R., Asghar, M.A., Asim, S., Khan, M.U., Hussain, R., ur Rehman, M.F., Ennis, C.J., Akram, M.S.: First principles study of electronic and nonlinear optical properties of A-D–π–A and D-A–D–π–A configured compounds containing novel quinoline–carbazole derivatives. RSC Adv. 10(37), 22273–22283 (2020a)
Khalid, M., Ali, A., Rehman, M.F.U., Mustaqeem, M., Ali, S., Khan, M.U., Asim, S., Ahmad, N., Saleem, M.: Exploration of noncovalent interactions, chemical reactivity, and nonlinear optical properties of piperidone derivatives: a concise theoretical approach. ACS Omega 5(22), 13236–13249 (2020b)
Khalid, M., Khan, M.U., Razia, E.-T., Shafiq, Z., Alam, M.M., Imran, M., Akram, M.S.: Exploration of efficient electron acceptors for organic solar cells: rational design of indacenodithiophene based non-fullerene compounds. Sci. Rep. 11(1), 1–15 (2021a)
Khalid, M., Lodhi, H.M., Khan, M.U., Imran, M.: Structural parameter-modulated nonlinear optical amplitude of acceptor–π–D–π–donor-configured pyrene derivatives: a DFT approach. RSC Adv. 11(23), 14237–14250 (2021b)
Khan, M.U., Khalid, M., Ibrahim, M., Braga, A.A.C., Safdar, M., Al-Saadi, A.A., Janjua, M.R.S.A.: First theoretical framework of triphenylamine–dicyanovinylene-based nonlinear optical dyes: structural modification of π-linkers. J. Phys. Chem. C 122(7), 4009–4018 (2018)
Khan, M.U., Ibrahim, M., Khalid, M., Braga, A.A.C., Ahmed, S., Sultan, A.: Prediction of second-order nonlinear optical properties of D–π–A compounds containing novel fluorene derivatives: a promising route to giant hyperpolarizabilities. J. Cluster Sci. 30(2), 415–430 (2019a)
Khan, M.U., Ibrahim, M., Khalid, M., Jamil, S., Al-Saadi, A.A., Janjua, M.R.S.A.: Quantum chemical designing of indolo [3, 2, 1-jk] carbazole-based dyes for highly efficient nonlinear optical properties. Chem. Phys. Lett. 719, 59–66 (2019b)
Khan, M.U., Ibrahim, M., Khalid, M., Qureshi, M.S., Gulzar, T., Zia, K.M., Al-Saadi, A.A., Janjua, M.R.S.A.: First theoretical probe for efficient enhancement of nonlinear optical properties of quinacridone based compounds through various modifications. Chem. Phys. Lett. 715, 222–230 (2019c)
Kovačević, N., Kokalj, A.: Analysis of molecular electronic structure of imidazole-and benzimidazole-based inhibitors: a simple recipe for qualitative estimation of chemical hardness. Corros. Sci. 53(3), 909–921 (2011)
Lesar, A., Milošev, I.: Density functional study of the corrosion inhibition properties of 1, 2, 4-triazole and its amino derivatives. Chem. Phys. Lett. 483(4–6), 198–203 (2009)
Lin, F., Jiang, K., Kaminsky, W., Zhu, Z., Jen, A.K.-Y.: A non-fullerene acceptor with enhanced intermolecular π-core interaction for high-performance organic solar cells. J. Am. Chem. Soc. 142(36), 15246–15251 (2020)
Liu, Q., Wang, Q., Xu, M., Liu, J., Liang, J.: DFT characterization and design of anthracene-based molecules for improving spectra and charge transfer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 227, 117627–117627 (2020)
Muhammad, S., Janjua, M.R.S.A., Su, Z.: Investigation of dibenzoboroles having π-electrons: toward a new type of two-dimensional NLO molecular switch? J. Phys. Chem. C 113(28), 12551–12557 (2009)
Muhammad, S., Xu, H.-L., Zhong, R.-L., Su, Z.-M., Al-Sehemi, A.G., Irfan, A.: Quantum chemical design of nonlinear optical materials by sp 2-hybridized carbon nanomaterials: issues and opportunities. J. Mater. Chem. C 1(35), 5439–5449 (2013)
Nagarajan, B., Kushwaha, S., Elumalai, R., Mandal, S., Ramanujam, K., Raghavachari, D.: Novel ethynyl-pyrene substituted phenothiazine based metal free organic dyes in DSSC with 12% conversion efficiency. J. Mater. Chem. A 5(21), 10289–10300 (2017)
Namuangruk, S., Fukuda, R., Ehara, M., Meeprasert, J., Khanasa, T., Morada, S., Kaewin, T., Jungsuttiwong, S., Sudyoadsuk, T., Promarak, V.: D-D− π–A-Type organic dyes for dye-sensitized solar cells with a potential for direct electron injection and a high extinction coefficient: synthesis, characterization, and theoretical investigation. J. Phys. Chem. C 116(49), 25653–25663 (2012)
Panneerselvam, M., Kathiravan, A., Solomon, R.V., Jaccob, M.: The role of π-linkers in tuning the optoelectronic properties of triphenylamine derivatives for solar cell applications–A DFT/TDDFT study. Phys. Chem. Chem. Phys. 19(8), 6153–6163 (2017)
Papadopoulos, M.G., Sadlej, A.J., Leszczynski, J.: Non-Linear Optical Properties of Matter. Springer (2006)
Parr, R.G., Donnelly, R.A., Levy, M., Palke, W.E.: Electronegativity: the density functional viewpoint. J. Chem. Phys. 68(8), 3801–3807 (1978)
Parr, R.G., Szentpály, L.V., Liu, S.: Electrophilicity index. J. Am. Chem. Soc. 121(9), 1922–1924 (1999)
Peng, Z., Yu, L.: Second-order nonlinear optical polyimide with high-temperature stability. Macromolecules 27(9), 2638–2640 (1994)
Prasad, P.N., Williams, D.J.: Introduction to Nonlinear Optical Effects in Molecules and Polymers, vol. 1. Wiley, New York (1991)
Qin, C., Clark, A.E.: DFT characterization of the optical and redox properties of natural pigments relevant to dye-sensitized solar cells. Chem. Phys. Lett. 438(1–3), 26–30 (2007)
Reed, A.E., Curtiss, L.A., Weinhold, F.: Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 88(6), 899–926 (1988)
Reis, H., Papadopoulos, M.G., Munn, R.: Calculation of macroscopic first-, second-, and third-order optical susceptibilities for the urea crystal. J. Chem. Phys. 109(16), 6828–6838 (1998)
Roy, R.S., Nandi, P.K.: Exploring bridging effect on first hyperpolarizability. RSC Adv. 5(125), 103729–103738 (2015)
Shahid, M., Salim, M., Khalid, M., Tahir, M.N., Khan, M.U., Braga, A.A.C.: Synthetic, XRD, non-covalent interactions and solvent dependent nonlinear optical studies of Sulfadiazine-Ortho-Vanillin Schiff base:(E)-4-((2-hydroxy-3-methoxy-benzylidene) amino)-N-(pyrimidin-2-yl) benzene-sulfonamide. J. Mol. Struct. 1161, 66–75 (2018)
Szafran, M., Komasa, A., Bartoszak-Adamska, E.: Crystal and molecular structure of 4-carboxypiperidinium chloride (4-piperidinecarboxylic acid hydrochloride). J. Mol. Struct. 827(1–3), 101–107 (2007)
Tamer, Ö., Avcı, D., Atalay, Y.: Synthesis, X-Ray crystal structure, photophysical characterization and nonlinear optical properties of the unique manganese complex with picolinate and 1, 10 phenantroline: toward the designing of new high NLO response crystal. J. Phys. Chem. Solids 99, 124–133 (2016)
Tsutsumi, N., Morishima, M., Sakai, W.: Nonlinear optical (NLO) polymers. 3. NLO polyimide with dipole moments aligned transverse to the imide linkage. Macromolecules 31(22), 7764–7769 (1998)
Valverde, C., e Castro, S.A., Vaz, G.R., de Almeida Ferreira, J.L., Baseia, B., Osório, F.A.: Third-order nonlinear optical properties of a carboxylic acid derivative. Acta Chim. Slov. 65(3), 739–749 (2018)
Wadsworth, A., Moser, M., Marks, A., Little, M.S., Gasparini, N., Brabec, C.J., Baran, D., McCulloch, I.: Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 48(6), 1596–1625 (2019)
Weinhold, F., Landis, C.R.: Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective. Cambridge University Press (2005)
Wielopolski, M., Kim, J.H., Jung, Y.S., Yu, Y.J., Kay, K.Y., Holcombe, T.W., Zakeeruddin, S.M., Grätzel, M., Moser, J.E.: Position-dependent extension of π-conjugation in D–π–A dye sensitizers and the impact on the charge-transfer properties. J. Phys. Chem. C 117(27), 13805–13815 (2013)
Yamashita, S.: A tutorial on nonlinear photonic applications of carbon nanotube and graphene. J. Lightwave Technol. 30(4), 427–447 (2011)
Zhang, B., Shi, G., Yang, Z., Zhang, F., Pan, S.: Fluorooxoborates: beryllium-free deep-ultraviolet nonlinear optical materials without layered growth. Angew. Chem. Int. Ed. 56(14), 3916–3919 (2017)
Zhurko, G., Zhurko, D.: ChemCraft, version 1.6 (2009). URL: http://www.chemcraftprog.com
Acknowledgements
Dr. Muhammad Khalid gratefully acknowledges the financial support of HEC Pakistan (project no. 20-14703/NRPU/R&D/HEC/2021). Authors are also thankful for cooperation and collaboration of A.A.C.B from IQ-USP, Brazil especially for his continuous support and providing computational lab facilities. S.F.A.M. acknowledges CNPq for the scholarship (Grant 165726/2020-2). A.A.C.B. (grants 2011/07895-8, 2015/01491-3, and 2014/25770-6) is highly thankful to Fundação de Amparo à Pesquisa do Estado de São Paulo for the cooperation and financial assistance. A.A.C.B. (grant 312550/2020-0) also thanks to the Brazilian National Research Council (CNPq) for financial support and fellowships.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MK: Methodology; software; project administration; SN: Data curation; formal analysis; MST: Resources; software; supervision; IS: Data curation; formal analysis; KSM: Conceptualization; resources; SFAM: Conceptualization; methodology; AACB: Data curation; formal analysis; validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalid, M., Naseer, S., Tahir, M.S. et al. A theoretical approach towards designing of banana shaped non-fullerene chromophores using efficient acceptors moieties: exploration of their NLO response properties. Opt Quant Electron 55, 258 (2023). https://doi.org/10.1007/s11082-022-04441-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-022-04441-w