Abstract
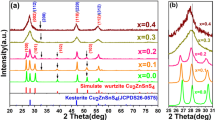
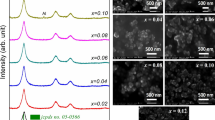
In this research, Ag+ and Al3+ doped CuInS2 (CIS) nanoparticles were synthesized by a low-cost and non-vacuum hydrothermal method. The CuInS2 nanoparticles were synthesized by the stoichiometric ratio of (1Cu: 1In: 2S) using the hydrothermal method at 180℃ and for 16, 18 and 20 h. Afterward, Ag+ and Al3+ ions were doped with non-stoichiometric composition of (Cu1-xAgx)InS2, Cu(In1-xAlx)S2 (x = 0.1, 0.2, and 0.3), and (Cu1-xAgx)(In1-xAlx)S2 (x = 0.1). The phase evolution, microstructural, and optical properties have also been studied by X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), UV–Vis spectroscopy techniques. The XRD results showed that the sample was prepared by the hydrothermal route at 180° C for 20 h was single-phase and no impurity phase was detected. However, some minor impurity phases (CuS and Cu9S5) were detected in the samples were synthesized at 180° C in 16 h and 18 h. The results show that the crystallite size of the synthesized nanoparticles of CIS at 16, 18, and 20 h are 26, 29, and 32 nm, respectively. The lattice parameters for the sample synthesized in 20 h were calculated to be 5.52 Å (a) and 11.11 Å (c). The FE-SEM micrographs showed that the pure CIS nanoparticles have flower-shaped morphology and the type and concentration of used dopants affect the morphology of the nanoparticles. The bandgap of single-phase CIS synthesized nanoparticles was 1.5 eV. However, the bandgap of (Cu0.9Ag0.1)InS2 and Cu(In0.9Al0.1)S2 was 1.4 eV and 1.70, respectively. The bandgap of (Cu0.9Ag0.1) (In0.9Al0.1)S2 was 2 eV.













Similar content being viewed by others
References
Aldakov, D., Lefrançois, A., Reiss, P.: Ternary and quaternary metal chalcogenide nanocrystals: synthesis, properties and applications. J. Mater. Chem. C. 1, 3756–3776 (2013)
Allouche, N., Kamoun, N., Jebbari, C.G., Kamoun Turki, N.: Influence of aluminum doping in CuInS2 prepared by spray pyrolysis on different substrates. J. Alloys Compd. 501, 85–88 (2010)
Benbelgacem, J., Ben Marai, A., Mendil, R., Medjnoun, K., Djessas, K., Ben Ayadi, Z.: Synthesis of CuInS2 nanoparticles by solvothermal process using dimethylformamide as a solvent. J. Alloys Compd. 692, 966–971 (2017)
Brik, M.G.: First-principles study of the electronic and optical properties of CuXS2 (X= Al, Ga, In) and AgGaS2 ternary compounds. JPCS. 21, 485–502 (2009)
Cui, Y., Ren, J., Chen, G., Qian, Y., Xie, Yi.: A simple route to synthesize MInS2 (M= Cu, Ag) nanorods from single-molecule precursors. Chem. Lett. 30, 236–237 (2001)
Delahoy, A.E., Guo, S., Luque, A., Hegedus, S.: Handbook of Photovoltaic Science and Engineering, pp. 716–796. Wiley, United Kingdom (2011)
Deng, W., Yan, Z., Fang, Y., Wang, Y.: Effect of Al content on the performance of Cu(In1− xAlx)S2 synthesized by hydrothermal solution processing. J Mater Sci: Mater Electron 25, 2829–2834 (2014)
Freitas, De., Jilian, N., Nogueira, A.F.: Incorporation of Inorganic Nanoparticles into Bulk Heterojunction Organic Solar Cells. In: Nanoenergy, pp. 1–47. Springer, Berlin, Heidelberg (2013)
Heidariramsheh, M., Dabbagh, M.M., Mahdavi, S.M., Beitollahi, A.: Morphology and phase-controlled growth of CuInS2 nanoparticles through polyol based heating up synthesis approach. Mater. Sci. Semicond. Process. 121, 105401 (2020)
Kavcar, N.: Study of the sub-bandgap absorption and the optical transitions in CuInSe2 polycrystalline thin films. Sol. Energy Mater. Sol. Cells. 52, 183–195 (1998)
Kim, Sung Jin. Nanostructured photovoltaic devices for next generation solar cell. State University of New York at Buffalo, 2008, 1–10.
Kolny-Olesiak, Joanna, and Horst Weller. Synthesis and application of colloidal CuInS2 semiconductor nanocrystals. ACShttps://www.google.com/search?rlz=1C1CHZL_enIR851IR851&q=ACS&stick=H4sIAAAAAAAAAONgVuLUz9U3MDJPKrNcxMrs6BwMAPLeEXATAAAA&sa=X&ved=2ahUKEwiiyfLd9LvjAhWEzKQKHVSYDEwQmxMoATAbegQICRAR Appl. Mater. Interfaces, 2013, 5, 12221–12237.
Partain, L.D., Fraas, L.M. (eds.): Solar cells and their applications, pp. 45–65. Hoboken, New Jersey (2010)
Saini, H.S., Singh, M., Reshak, A.H., Kashyap, M.K.: Effect of cation substitution on electronic band structure of ZnGeAs2 pnictides : A mBJLDA approach. J. Alloys Compd. 518, 74–79 (2012)
Tadjarodi, A., Cheshmekhavar, A.H., Imani, M.: Preparation of AgInS2 nanoparticles by a facile microwave Heating technique; study of effective parameters, optical and photovoltaic characteristics. Appl. Surf. Sci. 263, 449–456 (2012)
Yao, J., Rudyk, B.W., Brunetta, C.D., Knorr, K.B., Figore, H.A., Mar, A., Aitken, J.A.: Mn incorporation in CuInS2 chalcopyrites: Structure, magnetism and optical properties. Mater. Chem. Phys. 136, 415–423 (2012)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahimi, S., Mirkazemi, S.M. & beitollahi, A. The effects of Ag+ and Al3+ substitution in (Cu1-xAgx)(In1-x Alx)S2 chalcopyrite nanoparticles synthesized by hydrothermal method: Study of microstructures and optical properties. Opt Quant Electron 53, 115 (2021). https://doi.org/10.1007/s11082-021-02753-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-021-02753-x