Abstract
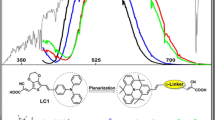
We designed and screened a series of metal-free organic dyes with different tunable donors (D1–D3) based on literature dye (3a). The D1–D3 organic dyes have been applied in dye-sensitized solar cells (DSSCs). Triphenylamine, thiophene and 2-cyanoacrylic acid groups as an electron donor (D), spacer (π) and electron acceptor (A), respectively, to form a D–π–A system. Density functional theory (DFT) and time-dependent DFT was employed to study the electronic charge distribution, intramolecular charge transfer, absorption spectra and photovoltaic (PV) parameters of the dyes. PV presentation of the dye molecules depends on tunable donors (D1–D3) classification. Results reveal that the replacing group of D3 dye has been red-shift absorption spectra and improves PV cell performance. Particularly, D2 and D3 dyes have smaller energy gaps and higher absorption spectra covering entire visible region, compared to D1 dye. Therefore, these results display that molecular tailoring is a promising scheme to improve D–π–A design of sensitizer for highly efficient DSSCs application.





Similar content being viewed by others
References
Adamo, C., Barone, V.: Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999)
Arunkumar, A., Anbarasan, P.M.: Highly efficient organic indolocarbazole dye in different acceptor units for optoelectronic applications—a first principle study. Struct. Chem. 29, 967–976 (2018)
Arunkumar, A., Anbarasan, P.M.: Optoelectronic properties of a simple metal-free organic sensitizer with different spacer groups: quantum chemical assessments. J. Electron. Mater. 48, 1522–1530 (2019)
Arunkumar, A., Prakasam, M., Anbarasan, P.M.: Influence of donor substitution at D–π–A architecture in efficient sensitizers for dye-sensitized solar cells: first principle study. Bull. Mater. Sci. 40, 1389–1396 (2017)
Arunkumar, A., Shanavas, S., Anbarasan, P.M.: First-principles study of efficient henothiazine-based D–π–A organic sensitizers with various spacers for DSSCs. J. Comput. Electron. 17, 1410–1420 (2018)
Asbury, J.B., Wang, Y.Q., Hao, E., Ghosh, H., Lian, T.: Evidences of hot excited state electron injection from sensitizer molecules to TiO2 nanocrystalline thin films. Res. Chem. Intermed. 27, 393–406 (2001)
Becke, A.D.: Density-functional thermochemistry: the role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Brian, O.R., Michael, G.: A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991)
Casanova, D., Rotzinger, F.P., Grätzel, M.: Computational study of promising organic dyes for high-performance sensitized solar cells. J. Chem. Theory Comput. 6, 1219–1227 (2010)
Chaitanya, K., Ju, X.-H., Heron, B.M.: Theoretical study on the light harvesting efficiency of zinc porphyrin sensitizers for DSSCs. RSC Adv. 4, 26621–26634 (2014)
Cossi, M., Barone, V.: Time-dependent density functional theory for molecules in liquid solutions. Chem. Phys. 115, 4708–4717 (2001)
Dreuw, A., Head-Gordon, M.: Single-reference ab initio methods for the calculation of excited states of large molecules. Chem. Rev. 105, 4009–4037 (2005)
Dualeh, A., De Angelis, F., Fantacci, S., Moehl, T., Yi, C., Kessler, F., Baranoff, E., Nazeeruddin, M.K., Grätzel, M.: Influence of donor groups of organic D–π–a dyes on open-circuit voltage in solid-state dye-sensitized solar cells. J. Phys. Chem. C 116, 1572–1578 (2011)
Duncan, W.R., Prezhdo, O.V.: Theoretical studies of photoinduced electron transfer in dye-sensitized TiO2. Annu. Rev. Phys. Chem. 58, 143–184 (2007)
Fernandes, S.S., Castro, M.C.R., Pereira, A.I., Mendes, A., Serpa, C., Pina, J., Justino, L.L., Burrows, H.D., Raposo, M.M.M.: Optical and photovoltaic properties of thieno [3, 2-b] thiophene-based push-pull organic dyes with different anchoring groups for dye-sensitized solar cells. ACS Omega 2, 9268–9279 (2017)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M.J., Heyd, J., Brothers, E.N., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A.P., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, N.J., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.J., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, Ö., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09. Gaussian Inc., Wallingford (2009)
Geskin, V.M., Lambert, C., Bredas, J.L.: Origin of high second-and third-order nonlinear optical response in ammonio/borato diphenylpolyene zwitterions: the remarkable role of polarized aromatic groups. J. Am. Chem. Soc. 125, 15651–15658 (2003)
Grätzel, M.: Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 42, 1788–1798 (2009)
Guichaoua, D., Kulyk, B., Smokal, V., Migalska-Zalas, A., Kharchenko, O., Krupka, O., Kolendo, O., Sahraoui, B.: UV irradiation induce NLO modulation in photochromic styrylquinoline-based polymers: computational and experimental studies. Organ. Electron. 66, 175–182 (2019)
Hagberg, D.P., Edvinsson, T., Marinado, T., Boschloo, G., Hagfeldt, A., Sun, L.: A novel organic chromophore for dye-sensitized nanostructured solar cells. Chem. Commun. 21, 2245–2247 (2006)
Hagfeldt, A., Graetzel, M.: Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 95, 49–68 (1995)
Hagfeldt, A., Boschloo, G., Sun, L.C., Kloo, L., Pettersson, H.: Dye-sensitized solar cells. Chem. Rev. 110, 6595–6663 (2010)
Hart, A.S., KC, C.B., Subbaiyan, N.K., Karr, P.A., D’Souza, F.: Phenothiazine-sensitized organic solar cells: effect of dye anchor group positioning on the cell performance. ACS Appl. Mater. Interfaces 4, 5813–5820 (2012)
Islam, A., Sugihara, H., Arakawa, H.: Molecular design of ruthenium (II) polypyridyl photosensitizers for efficient nanocrystalline TiO2 solar cells. J. Photochem. Photobio A: Chem. 158, 131–138 (2003)
Ito, S., Zakeeruddin, S.M., Humphry-Baker, R., Liska, P., Charvet, R., Comte, P., Nazeeruddin, M.K., Péchy, P., Takata, M., Miura, H., Uchida, S., Grätzel, M.: High-efficiency organic-dye-sensitized solar cells controlled by nanocrystalline-TiO2 electrode thickness. Adv. Mater. 18, 1202–1205 (2006)
Jungsuttiwong, S., Tarsang, R., Sudyoadsuk, T., Promarak, V., Khongpracha, P., Namuangruk, S.: Theoretical study on novel double donor-based dyes used in high efficient dye-sensitized solar cells: the application of TDDFT study to the electron injection process. Organ. Electron. 14, 711–722 (2013)
Kakiage, K., Aoyama, Y., Yano, T., Oya, K., Fujisama, J., Hanaya, M.: Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 51, 15894–15896 (2015)
Kulyk, B., Taboukhat, S., Akdas-Kilig, H., Fillaut, J.L., Boughaleb, Y., Sahraoui, B.: Nonlinear refraction and absorption activity of dimethylaminostyryl substituted BODIPY dyes. RSC Adv. 6, 84854–84859 (2016)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988)
Li, Y., Pullerits, T., Zhao, M., Sun, M.: Theoretical characterization of the PC60BM: PDDTT model for an organic solar cell. J. Phys. Chem. C 115, 21865–21873 (2011)
Li, M., Kou, L., Diao, L., Zhang, Q., Li, Z., Wu, Q., Lu, W., Pan, D., Wei, Z.: Theoretical study of WS-9-Based organic sensitizers for unusual vis/NIR absorption and highly efficient dye-sensitized solar cells. J. Phys. Chem. C 119, 9782–9790 (2015)
Liang, M., Chen, J.: Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 42, 3453–3488 (2013)
Lin, Y.S., Li, G.D., Mao, S.P., Chai, J.D.: Long-range corrected hybrid density functionals with improved dispersion corrections. J. Chem. Theory Comput. 9, 263–272 (2012)
Martsinovich, N., Troisi, A.: Theoretical studies of dye-sensitised solar cells: from electronic structure to elementary processes. Energy Environ. Sci. 4, 4473–4495 (2011)
Mathew, S., Imahori, H.: Tunable, strongly-donating perylene photosensitizers for dye-sensitized solar cells. J. Mater. Chem. 21, 7166–7174 (2011)
Mba, M., D’Acunzo, M., Salice, P., Carofiglio, T., Maggini, M., Caramori, S., Campana, A., Aliprandi, A., Argazzi, R., Carli, S., Bignozzi, C.A.: Sensitization of nanocrystalline TiO2 with multibranched organic dyes and Co (III)/(II) mediators: strategies to improve charge collection efficiency. J. Phys. Chem. C 117, 19885–19896 (2013)
Migalska-Zalas, A., Korchi, K.E., Chtouki, T.: Enhanced nonlinear optical properties due to electronic delocalization in conjugated benzodifuran derivatives. Opt. Quant. Electron. 50, 389–398 (2018)
Mishra, A., Fischer, M.K.R., Bäuerle, P.: Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. 48, 2474–2499 (2009)
Nakano, M., Fujita, H., Takahata, M., Yamaguchi, K.: Theoretical study on second hyperpolarizabilities of phenylacetylene dendrimer: toward an understanding of structure-property relation in NLO responses of fractal antenna dendrimers. J. Am. Chem. Soc. 124, 9648–9655 (2002)
Nithya, R., Senthilkumar, K.: Theoretical studies on the quinoidal thiophene based dyes for dye sensitized solar cell and NLO applications. Phys. Chem. Chem. Phy. 16, 21496–21505 (2014)
O’boyle, N.M., Tenderholt, A.L., Langner, K.M.: Cclib: a library for package-independent computational chemistry algorithms. J. Comput. Chem. 29, 839–845 (2008)
Ren, X.M., Jiang, S.H., Cha, M.Y., Zhou, G., Wang, Z.-S.: Thiophene-bridged double D–π–A dye for efficient dye-sensitized solar cell. Chem. Mater. 24, 3493–3499 (2012)
Sayama, K., Tsukagoshi, S., Mori, T., Hara, K., Ohga, Y., Shinpou, A., Abe, Y., Suga, S., Arakawa, H.: Efficient sensitization of nanocrystalline TiO2 films with cyanine and merocyanine organic dyes. Sol. Energy Mater. Sol. Cells 80, 47–71 (2003)
Tarsang, R., Promarak, V., Sudyoadsuk, T., Namuangruk, S., Kungwan, N., Khongpracha, P., Jungsuttiwong, S.: Triple bond-modified anthracene sensitizers for dye-sensitized solar cells: a computational study. RSC Adv. 5, 38130–38140 (2015)
Wang, Z.S., Hara, K., Dan-oh, Y., Kasada, C., Shinpo, A., Suga, S., Arakawa, H., Sugihara, H.: Photophysical and (photo) electrochemical properties of a coumarin dye. J. Phys. Chem. B 109, 3907–3914 (2005)
Wang, Z.S., Koumura, N., Cui, Y., Takahashi, M., Sekiguchi, H., Mori, A., Kubo, T., Furube, A., Hara, K.: Hexylthiophene-functionalized carbazole dyes for efficient molecular photovoltaics: tuning of solar-cell performance by structural modification. Chem. Mater. 20, 3993–4003 (2008)
Yanai, T., Tew, D.P., Handy, N.C.: A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004)
Yella, A., Lee, H.W., Tsao, H.N., Yi, C., Chandiran, A.K., Nazeeruddin, M.K., Diau, E.W.G., Yeh, C.Y., Zakeeruddin, S.M., Grätzel, M.: Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 334, 629–634 (2011)
Zawadzka, A., Waszkowska, K., Karakas, A., Płóciennik, P., Korcala, A., Wisniewski, K., Karakaya, M., Sahraoui, B.: Diagnostic and control of linear and nonlinear optical effects in selected self-assembled metallophthalocyanine chlorides nanostructures. Dyes Pigm. 157, 151–162 (2018)
Zeng, W., Cao, Y., Bai, Y., Wang, Y., Shi, Y., Zhang, M., Wang, F., Pan, C., Wang, P.: Efficient dye-sensitized solar cells with an organic photosensitizer featuring orderly conjugated ethylenedioxythiophene and dithienosilole blocks. Chem. Mater. 22, 1915–1925 (2010)
Zhang, J., Kan, Y.-H., Li, H.-B., Geng, Y., Wu, Y., Su, Z.-M.: How to design proper π-Spacer order of the D–π–A dyes for DSSCs? A density functional response. Dyes Pigm. 95, 313–321 (2012)
Zhang, Z.L., Zou, L.Y., Ren, A.M., Liu, Y.F., Feng, J.K., Sun, C.C.: Theoretical studies on the electronic structures and optical properties of star-shaped triazatruxene/heterofluorene co-polymers. Dyes Pigm. 96, 349–363 (2013)
Zhao, Y., Liang, W.: Charge transfer in organic molecules for solar cells: theoretical perspective. Chem. Soc. Rev. 41, 1075–1087 (2012)
Zhu, H., Li, W., Wu, Y., Liu, B., Zhu, S., Li, X., Ågren, H., Zhu, W.: Insight into benzothiadiazole acceptor in D–A–π–A configuration on photovoltaic performances of dye-sensitized solar cells. ACS Sustain. Chem. Eng. 2, 1026–1034 (2014)
Acknowledgements
The authors are thankful to the learned referees for their useful and critical comments, which can be improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S0
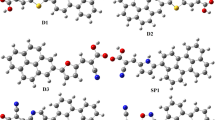
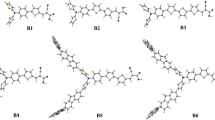
Optimized geometric structures of the 3a and designed dye molecules were calculated by using B3LYP/6-31G(d) theory. (DOC 112 kb)
Rights and permissions
About this article
Cite this article
Arunkumar, A., Shanavas, S., Acevedo, R. et al. Computational analysis on D–π–A based perylene organic efficient sensitizer in dye-sensitized solar cells. Opt Quant Electron 52, 164 (2020). https://doi.org/10.1007/s11082-020-02273-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-020-02273-0