Abstract
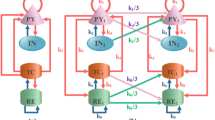
The mechanisms of network and transition dynamics of epileptiform activity remain unclear. In general, the transitions of epileptiform discharges comprise slow interictal discharges, ictal discharges and postictal depression. Studies have indicated that network properties and the inherent parameters of neuronal models have great impacts on the transitions. Recently, a novel neuromodulation technique, transcranial magneto-acoustical stimulation (TMAS), has been tested for its efficiency experimentally and computationally. In this paper, we establish a biophysical computational network model of an ictogenic hippocampus area to investigate the underlying transitions mechanisms and reveal neuromodulation mechanisms combined with TMAS. Results demonstrate that long distance connections caused by increased connection probability and the number of nearest-neighbour edges make the network more random and focused. The cooperation of network topological structure and neuronal parameters including ion concentration and inherent external input of neurons could induce epileptic transitions. Moreover, the focused ultrasound transducer has the ability to launch and focus the transcranial ultrasound wave to the hippocampal area in the depth of the three-layer tissue. By coupling with a static magnetic field, the proposed modulated induced TMAS currents can terminate epileptiform activity but consumes more energy by regulating magnetic strength. However, changing modulation frequency was unable to fully suppress seizures. These computational results offer an explanation of the mechanisms of neurodynamics of epileptiform discharges and its neuromodulation by TMAS.










Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Haut, S.R., Nabbout, R.: Recognizing seizure clusters in the community: the path to uniformity and individualization in nomenclature and definition. Epilepsia 63(Suppl. 1), S6–S13 (2022). https://doi.org/10.1111/epi.17346
Sainburg, L.E., Janson, A.P., Johnson, G.W., et al.: Structural disconnection relates to functional changes after temporal lobe epilepsy surgery. Brain (2023). https://doi.org/10.1093/brain/awad117
Bernhardt, B.C., Bonilha, L., Gross, D.W.: Network analysis for a network disorder: the emerging role of graph theory in the study of epilepsy. Epilepsy Behav. Behav. 50, 162–170 (2015). https://doi.org/10.1016/j.yebeh.2015.06.005
Voets, N.L., Beckmann, C.F., Cole, D.M., et al.: Structural substrates for resting network disruption in temporal lobe epilepsy. Brain 135, 2350–2357 (2012). https://doi.org/10.1093/brain/aws137
Watts, D.J., Strogatz, S.H.: Colletive dynamics of ‘small-world’ networks. Nature 393(4), 440–442 (1998). https://doi.org/10.1038/30918
Netoff, T.I., Clewley, R., Arno, S., et al.: Epilepsy in small-world networks. J. Neurosci.Neurosci. 24(37), 8075–8083 (2004). https://doi.org/10.1523/JNEUROSCI.1509-04.2004
Percha, B., Dzakpasu, R., Żochowski, M.: Transition from local to global phase synchrony in small world neural network and its possible implications for epilepsy. Phys. Rev. E 72, 031909 (2005). https://doi.org/10.1103/PhysRevE.72.031909
Scharfman, H.E.: The enigmatic mossy cell of the dentate gyrus. Nat. Rev. Neurosci.Neurosci. 17, 562–575 (2016). https://doi.org/10.1038/nrn.2016.87
Shiri, Z., Manseau, F., Lévesque, M., et al.: Activation of specific neuronal networks leads to different seizure onset types. Ann. Neurol. 79(3), 354–365 (2016). https://doi.org/10.1002/ana.24570
Cordon, T., English, A.W.: Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur. J. Neurosci.Neurosci. 43(3), 336–350 (2016). https://doi.org/10.1111/ejn.13005
Zhang, L., Fan, D., Wang, Q.: Synchronous high-frequency oscillations in inhibitory-dominant network motifs consisting of three dentate gyrus-CA3 systems. Chaos 28, 063101 (2018). https://doi.org/10.1063/1.5017012
Zhang, L., Ma, Z., Yu, Y., et al.: Examining the low-voltage fast seizure-onset and its response to optogenetic stimulation in a biophysical network model of the hippocampus. Cogn. Neurodyn.. Neurodyn. (2023). https://doi.org/10.1007/s11571-023-09935-1
Yu, Y., Han, F., Wang, Q.: A hippocampal-entorhinal cortex neuronal network for dynamical mechanisms of epileptic seizure. IEEE Trans. Neural Syst. Rehabil. Eng.Rehabil. Eng. 31, 1986–1996 (2022). https://doi.org/10.1109/TNSRE.2023.3265581
Ahn, S., Jun, S.B., Lee, H.W., et al.: Computational modeling of epileptiform activities in medial temporal lobe epilepsy combined with in vitro experiments. J. Comput. Neurosci.Comput. Neurosci. 41, 207–223 (2016). https://doi.org/10.1007/s10827-016-0614-8
Wendling, F., Benquet, P., Bartolomei, F., et al.: Computational models of epileptiform activity. J. Neurosci. MethodsNeurosci. Methods 260, 233–251 (2016). https://doi.org/10.1016/j.jneumeth.2015.03.027
Curia, G., Longo, D., Biagini, G., et al.: The pilocarpine model of temporal lobe epilepsy. J. Neurosci. MethodsNeurosci. Methods 172, 143–157 (2008). https://doi.org/10.1016/j.jneumeth.2008.04.019
Yu, Y., Hao, Y., Wang, Q.: Model-based optimized phase-deviation deep brain stimulation for Parkinson’s disease. Neural Netw.Netw. 122, 308–319 (2019). https://doi.org/10.1016/j.neunet.2019.11.001
Ben-Menachem, E.: Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 1, 477–482 (2002). https://doi.org/10.1016/S1474-4422(02)00220-X
Yang, A.-C., Shi, L., Li, L.-M., et al.: Potential protective effects of chronic anterior thalamic nucleus stimulation on hippocampal neurons in epileptic monkeys. Brain Stimul.Stimul. 8, 1049–1057 (2015). https://doi.org/10.1016/j.brs.2015.07.041
Krook-Magnuson, E., Szabo, G.G., Armstrong, C., et al.: Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro 1(1), e.2014 (2014). https://doi.org/10.1523/ENEURO.0005-14.2014
Regner, G.G., Pereira, P., Leffa, D.T., et al.: Preclinical to clinical translation of studies of transcranial direct-current stimulation in the treatment of epilepsy: a systematic review. Front. Neurosci.Neurosci. 12, 189 (2018). https://doi.org/10.3389/fnins.2018.00189
Rabut, C., Yoo, S., Hurt, R.C., et al.: Ultrasound technologies for imaging and modulating neural activity. Neuron 108, 93–110 (2020). https://doi.org/10.1016/j.neuron.2020.09.003
Brinker, S.T., Preiswerk, F., White, P.J., et al.: Focused ultrasound platform for investigating therapeutic neuromodulation across the human hippocampus. Ultrasound Med. Biol. 46(5), 1270–1274 (2020). https://doi.org/10.1016/j.ultrasmedbio.2020.01.007
Zhang, H., Yu, Y., Deng, Z., et al.: Activity pattern analysis of the subthalamopallidal network under ChannelRhodopsin-2 and Halorhodopsin photocurrent control. Chaos Soliton Fract. 138, 109963 (2020). https://doi.org/10.1016/j.chaos.2020.109963
Zhao, J., Yu, Y., Wang, Q.: Dynamical regulation of epileptiform discharges caused by abnormal astrocyte function with optogenetic stimulation. Chaos Soliton Fract. 164, 112720 (2022). https://doi.org/10.1016/j.chaos.2022.112720
Norton, S.J.: Can ultrasound be used to stimulate nerve tissue. Biomed. Eng. 2, 6 (2003). https://doi.org/10.1186/1475-925X-2-6
Zhang, Y., Zhang, M., Ling, Z., et al.: The influence of transcranial magnetoacoustic stimulation parameters on the basal ganglia-thalamus neural network in Parkinson’s disease. Front. Neuosci. 15, 761720 (2021). https://doi.org/10.3389/fnins.2021.761720
Yuan, Y., Pang, N., Chen, Y., et al.: A phase-locking analysis of neuronal firing rhythms with transcranial magneto-acoustical stimulation based on the Hodgkin–Huxley neuron model. Front. Comput. Neurosci.Comput. Neurosci. 11, 1 (2017). https://doi.org/10.3389/fncom.2017.00001
Zhou, X., Liu, S., Wang, Y., et al.: High-resolution transcranial electrical stimulation for living mice based on magneto-acoustic effect. Front. Neurosci.Neurosci. 13, 1342 (2019). https://doi.org/10.3389/fnins.2019.01342
McLean, M.J., Engström, S., Zhang, Q., et al.: Effects of a static magnetic filed on audiogenic seizures in black Swiss mice. Epilepsy Res. 80(2–3), 119–131 (2008). https://doi.org/10.1016/j.eplepsyres.2008.03.022
Qiu, Z., Kala, S., Guo, J., et al.: Targeted neurostimulation in mouse brains with non-invasive ultrasound. Cell Rep. 32(7), 108033 (2020). https://doi.org/10.1016/j.celrep.2020.108033
Olufsen, M., Whittington, M., Camperi, M., et al.: New functions for the gamma rhythm: population tuning and preprocessing for the beta rhythm. J. Comput. Neurosci.Comput. Neurosci. 14(1), 33–54 (2003). https://doi.org/10.1023/A:1021124317706
Hodgkin, A.L., Huxley, A.F.: Currents carried by sodium and potassium ions through the membrane of the giant axon of loligo. J. Physiol. 116(4), 449–472 (1952). https://doi.org/10.1113/jphysiol.1952.sp004717
Wang, X., Buzśaki, G.: Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J. Neurosci.Neurosci. 16(20), 6402–6413 (1996). https://doi.org/10.1523/JNEUROSCI.16-20-06402.1996
Kopell, N., Börgers, C., Pervouchine, D., et al.: Gamma and theta rhythms in biophysical models of hippocampal circuits. In: Cutsuridis, V., Graham, B., Cobb, S., et al. (eds.) Hippocampal Microcircuits. Springer Series in Computational Neuroscience, vol. 5, pp. 423–457. Springer, New York (2010)
Bernhardt, B.C., Chen, Z., He, Y., et al.: Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb. Cortex. Cortex 21, 2147–2157 (2011). https://doi.org/10.1093/cercor/bhq291
Montalibet, A., Jossinet, J., Matias, A., Cathignol, D.: Electric current generated by ultrasonically induced Lorentz force in biological media. Med. Biol. Eng. Comput.Comput. 39, 15–20 (2001). https://doi.org/10.1007/BF02345261
Yuan, Y., Chen, Y., Li, X.: Theoretic analysis of transcranial magneto-acoustical stimulation with Hodgkin–Huxley neuron model. Front. Comput. Neurosci.Comput. Neurosci. 10, 35 (2016). https://doi.org/10.3389/fncom.2016.00035
Hendee, W.R., Ritenour, E.R.: Medical Imaging Physics, 4th edn. Wiley, New York (2002)
Treeby, B.E., Cox, B.T.: k-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave fields. J. Biomed. Opt. 15(2), 021314 (2010). https://doi.org/10.1117/1.3360308
Tufail, Y., Yoshihiro, A., Pati, S., et al.: Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc.Protoc. 6, 1453–1470 (2011). https://doi.org/10.1038/nprot.2011.371
Gnatkovsky, V., Librizzi, L., Trombin, F., et al.: Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann. Neurol. 64, 674–686 (2008). https://doi.org/10.1002/ana.21519
Magloire, V., Savtchenko, L.P., Jensen, T.P., et al.: Volume-transmitted GABA waves pace epileptiform rhythms in the hippocampal network. Curr. Biol. 33, 1–16 (2023). https://doi.org/10.1016/j.cub.2023.02.051
Barrio, R., Ibáñez, S., Pérez, L., et al.: Spike-adding structure in fold/hom bursters. Commun. Nonlinear Sci. Numer. Simul.. Nonlinear Sci. Numer. Simul. 83, 105100 (2020). https://doi.org/10.1016/j.cnsns.2019.105100
Barreto, E., Cressman, J.R.: Ion concentration dynamics as a mechanism for neuronal bursting. J. Biol. Phys. 37, 361–373 (2010). https://doi.org/10.1007/s10867-010-9212-6
Florence, G., Pereira, T., Kurth, J.: Extracellular potassium dynamics in the hyperexcitable state of the neuronal ictal activity. Commun. Nonlinear Sci. Numer. Simul.. Nonlinear Sci. Numer. Simul. 17, 4700–4706 (2012). https://doi.org/10.1016/j.cnsns.2011.06.023
Howe, T., Blockeel, A.J., Taylor, H., et al.: NMDA receptors promote hippocampal sharp-wave ripples and the associated coactivity of CA1 pyramidal cells. Hippocampus 30, 1356–1370 (2020). https://doi.org/10.1002/hipo.23276
Wang, Y., Feng, L., Liu, S., et al.: Transcranial magneto-acoustic stimulation improves neuroplasticity in hippocampus Parkinson’s disease model mice. Neurotherapeutics 16(4), 1210–1224 (2019). https://doi.org/10.1007/s13311-019-00732-5
Liu, R., Ma, R., Liu, X., et al.: A noninvasive deep brain stimulation method via temporal-spatial interference magneto-acoustic effects: simulation and experimental validation. IEEE Trans. Ultrason. Ferroelectr. Freq. ControlUltrason. Ferroelectr. Freq. Control 69(8), 2474–2483 (2022). https://doi.org/10.1109/TUFFC.2022.3187748
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 12102014, 11932003, 32271361 and 12202022) and the National Key Research and Development Program of China (Grant Nos. 2021YFA1000200 and 2021YFA1000202).
Author information
Authors and Affiliations
Contributions
LZ: Conceptualization, Methodology, Software, Writing original draft, Writing-reviewing and editing. YX: Designed, Performed the research, Analyzed the data, Writing the original draft. GB: Conceptualization, Methodology, Writing-reviewing and editing. YL: Conceptualization, Writing-reviewing. BL: Conceptualization, Methodology, Software, Writing-reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Xu, Y., Baier, G. et al. Exploring the dynamical transitions on an epileptic hippocampal network model and its modulation strategy based on transcranial magneto-acoustical stimulation. Nonlinear Dyn (2024). https://doi.org/10.1007/s11071-024-09476-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11071-024-09476-0