Abstract
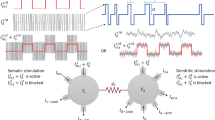
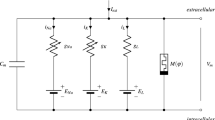
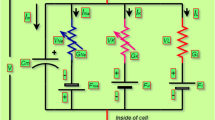
Due to the huge storage capacity and complex nonlinearity associated with the memristor or memory resistor, various modified single-compartment neuron models with a flux controlled memristor for exploring the influence of electromagnetic induction on dynamical response, including spiking patterns, have been presented. This paper generalizes the relevant investigation of the electrophysiology of a hippocampal CA3 pyramidal neuron by an electromagnetic induction-based variant of the two-compartment Pinsky–Rinzel neuron model. Our emphasis was on the uncertainty quantification and sensitivity analysis of ionic channel conductivities under the electromagnetic induction effect when the two-compartment model is near the Hopf bifurcation point. It was found that the quantities of interest (QoI), such as average interspike interval and spike frequency, mainly depend on the conductivities of calcium and calcium-activated potassium channels and their mutual interactions within a periodic bursting electrical mode. In the case of the aperiodic bursting electrical mode, these QoIs are most sensitive to the conductivities of potassium delayed rectifier, calcium, calcium-activated potassium channels, and their mutual interactions. Our computational results demonstrate that the electrical activities of the hippocampal CA3 pyramidal neuron model under the influence of magnetic flux are sensitive to the transition between complex periodic and aperiodic bursting electrical modes.















Similar content being viewed by others
Data availability statement
The data used to generate the numerical results of the current work are available from the corresponding author on request.
References
Kim, H., Sah, M.P., Yang, C., Cho, S., Chua, L.O.: Memristor emulator for memristor circuit applications. IEEE Trans. Circuits Syst. I Regul. Pap. 59(10), 2422–2431 (2012). https://doi.org/10.1109/TCSI.2012.2188957
Hu, C., Zuo, H., Li, Y.: Effects of radiofrequency electromagnetic radiation on neurotransmitters in the brain. Front. Public Health. (2021). https://doi.org/10.3389/fpubh.2021.691880
Taki, M., Watanabe, S.: Biological and health effects of exposure to electromagnetic field from mobile communications systems. IATSS Res. 25(2), 40–50 (2001). https://doi.org/10.1016/S0386-1112(14)60069-8
Capelli, E., Torrisi, F., Venturini, L., Granato, M., Fassina, L., Lupo, G.F.D., Ricevuti, G.: Low-frequency pulsed electromagnetic field is able to modulate miRNAs in an experimental cell model of Alzheimer’s disease. J. Healthc. Eng. (2017). https://doi.org/10.1155/2017/2530270
Fisher, R., Salanova, V., Witt, T.: Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51(5), 899–908 (2010). https://doi.org/10.1111/j.1528-1167.2010.02536.x
Qu, L., Du, L., Hu, H., Cao, Z., Deng, Z.: Pattern control of external electromagnetic stimulation to neuronal networks. Nonlinear Dyn. 102(4), 2739–2757 (2020). https://doi.org/10.1007/s11071-020-06076-6
Lin, H., Wang, C., Cui, L., Sun, Y., Zhang, X., Yao, W.: Hyperchaotic memristive ring neural network and application in medical image encryption. Nonlinear Dyn. 110(1), 841–855 (2022). https://doi.org/10.1007/s11071-022-07630-0
Li, K., Bao, H., Li, H., Ma, J., Hua, Z., Bao, B.: Memristive Rulkov neuron model with magnetic induction effects. IEEE Trans. Ind. Inform. 18(3), 1726–1736 (2021). https://doi.org/10.1109/TII.2021.3086819
Lin, H., Wang, C., Cui, L., Sun, Y., Xu, C., Yu, F.: Brain-like initial-boosted hyperchaos and application in biomedical image encryption. IEEE Trans. Ind. Inform. (2022). https://doi.org/10.1109/TII.2022.3155599
Lin, H., Wang, C., Xu, C., Zhang, X., Iu, H.H.: A memristive synapse control method to generate diversified multi-structure chaotic attractors. IEEE Trans. Comput. Aided Des. Integr. Circuit Syst. (2022). https://doi.org/10.1109/TCAD.2022.3186516
Chua, L.: Memristor-the missing circuit element. IEEE Trans. Circuit Theory 18(5), 507–519 (1971). https://doi.org/10.1109/TCT.1971.1083337
Strukov, D.B., Snider, G.S., Stewart, D.R., Williams, R.S.: The missing memristor found. Nature 453(7191), 80–83 (2008). https://doi.org/10.1038/nature06932
Joglekar, Y.N., Wolf, S.J.: The elusive memristor: properties of basic electrical circuits. Eur. J. Phys. 30(4), 661–675 (2009). https://doi.org/10.1088/0143-0807/30/4/001
Rajagopal, K., Jafari, S., Moroz, I., Karthikeyan, A., Srinivasan, A.: Noise induced suppression of spiral waves in a hybrid Fitzhugh–Nagumo neuron with discontinuous resetting. Chaos (2021). https://doi.org/10.1063/5.0059175
Zhan, F., Liu, S.: Response of electrical activity in an improved neuron model under electromagnetic radiation and noise. Front. Comput. Neurosci. (2017). https://doi.org/10.3389/fncom.2017.00107
Wang, Y., Ma, J., Xu, Y., Wu, F., Zhou, P.: The electrical activity of neurons subject to electromagnetic induction and gaussian white noise. Int. J. Bifurcat. Chaos 27(02), 1750030 (2017). https://doi.org/10.1142/S0218127417500304
Lv, M., Wang, C., Ren, G., Ma, J., Song, X.: Model of electrical activity in a neuron under magnetic flow effect. Nonlinear Dyn. 85(3), 1479–1490 (2016). https://doi.org/10.1007/s11071-016-2773-6
Lv, M., Ma, J.: Multiple modes of electrical activities in a new neuron model under electromagnetic radiation. Neurocomputing 205(C), 375–381 (2016). https://doi.org/10.1016/j.neucom.2016.05.004
Lu, L., Jia, Y., Liu, W., Yang, L.: Mixed stimulus-induced mode selection in neural activity driven by high and low frequency current under electromagnetic radiation. Complexity (2017). https://doi.org/10.1155/2017/7628537
Wu, J., Jin, M., Qiao, Q.: Modeling electrical stimulation of retinal ganglion cell with optimizing additive noises for reducing threshold and energy consumption. BioMed. Eng. Online (2017). https://doi.org/10.1186/s12938-017-0333-z
Fu, Y.-X., Kang, Y.-M., Xie, Y.: Subcritical Hopf bifurcation and stochastic resonance of electrical activities in neuron under electromagnetic induction. Front. Comput. Neurosci. (2018). https://doi.org/10.3389/fncom.2018.00006
Kafraj, M.S., Parastesh, F., Jafari, S.: Firing patterns of an improved Izhikevich neuron model under the effect of electromagnetic induction and noise. Chaos, Solitons Fractals 137, 109782 (2020). https://doi.org/10.1016/j.chaos.2020.109782
Yang, Y., Ma, J., Xu, Y., Jia, Y.: Energy dependence on discharge mode of Izhikevich neuron driven by external stimulus under electromagnetic induction. Cogn. Neurodyn. 15(2), 265–277 (2021). https://doi.org/10.1007/s11571-020-09596-4
Thompson, C.L., Pathak, S.D., Jeromin, A., Ng, L.L., MacPherson, C.R., Mortrud, M.T., Cusick, A., Riley, Z.L., Sunkin, S.M., Bernard, A., Puchalski, R.B., Gage, F.H., Jones, A.R., Bajic, V.B., Hawrylycz, M.J., Lein, E.S.: Genomic anatomy of the hippocampus. Neuron 60(6), 1010–1021 (2008). https://doi.org/10.1016/j.neuron.2008.12.008
Eichenbaum, H.: The hippocampus as a cognitive map ... of social space. Neuron 87(1), 9–11 (2015). https://doi.org/10.1016/j.neuron.2015.06.013
Raus Balind, S., Magó, Á., Ahmadi, M., Kis, N., Varga-Németh, Z., Lőrincz, A., Makara, J.K.: Diverse synaptic and dendritic mechanisms of complex spike burst generation in hippocampal CA3 pyramidal cells. Nat. Commun. 10(1), 1–15 (2019). https://doi.org/10.1038/s41467-019-09767-w
Traub, R.D., Wong, R.K., Miles, R., Michelson, H.: A model of a CA3 hippocampal pyramidal neuron incorporating voltage-clamp data on intrinsic conductances. J. Neurophysiol. 66(2), 635–650 (1991). https://doi.org/10.1152/jn.1991.66.2.635
Pinsky, P.F., Rinzel, J.: Intrinsic and network rhythmogenesis in a reduced Traub model for CA3 neurons. J. Comput. Neurosci. 1(1), 39–60 (1994). https://doi.org/10.1007/BF00962717
Mäki-Marttunen, T., Halnes, G., Devor, A., Metzner, C., Dale, A.M., Andreassen, O.A., Einevoll, G.T.: A stepwise neuron model fitting procedure designed for recordings with high spatial resolution: application to layer 5 pyramidal cells. J. Neurosci. Methods 293, 264–283 (2018). https://doi.org/10.1016/j.jneumeth.2017.10.007
Hay, E., Hill, S., Schürmann, F., Markram, H., Segev, I.: Models of neocortical layer 5b pyramidal cells capturing a wide range of dendritic and perisomatic active properties. PLoS Comput. Biol. 7(7), 1002107 (2011). https://doi.org/10.1371/journal.pcbi.1002107
Atherton, L.A., Prince, L.Y., Tsaneva-Atanasova, K.: Bifurcation analysis of a two-compartment hippocampal pyramidal cell model. J. Comput. Neurosci. 41(1), 91–106 (2016). https://doi.org/10.1007/s10827-016-0606-8
Ghori, M.B., Kang, Y., Chen, Y.: Emergence of stochastic resonance in a two-compartment hippocampal pyramidal neuron model. J. Comput. Neurosci. 50(2), 217–240 (2022). https://doi.org/10.1007/s10827-021-00808-2
Reznik, R.I., Barreto, E., Sander, E., So, P.: Effects of polarization induced by non-weak electric fields on the excitability of elongated neurons with active dendrites. J. Comput. Neurosci. 40(1), 27–50 (2016). https://doi.org/10.1007/s10827-015-0582-4
Wei, X., Chen, Y., Lu, M., Deng, B., Yu, H., Wang, J., Che, Y., Han, C.: An ephaptic transmission model of ca3 pyramidal cells: An investigation into electric field effects. Cogn. Neurodyn. 8(3), 177–197 (2014). https://doi.org/10.1007/s11571-013-9269-6
Sætra, M.J., Einevoll, G.T., Halnes, G.: An electrodiffusive, ion conserving Pinsky–Rinzel model with homeostatic mechanisms. PLoS Comput. Biol. 16(4), 1–36 (2020). https://doi.org/10.1371/journal.pcbi.1007661
Torres Valderrama, A., Witteveen, J., Navarro, M., Blom, J.: Uncertainty propagation in nerve impulses through the action potential mechanism. J. Math. Neurosci. 5(1), 1–9 (2015). https://doi.org/10.1186/2190-8567-5-3
Lojić Kapetanović, A., Šušnjara, A., Poljak, D.: Stochastic analysis of the electromagnetic induction effect on a neuron’s action potential dynamics. Nonlinear Dyn. 105(4), 3585–3602 (2021). https://doi.org/10.1007/s11071-021-06762-z
Hodgkin, A.L., Huxley, A.F.: A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117(4), 500–544 (1952). https://doi.org/10.1113/jphysiol.1952.sp004764
Rezvani-Ardakani, S., Mohammad-Ali-Nezhad, S., Ghasemi, R.: Epilepsy control using a fixed time integral super twisting sliding mode control for Pinsky–Rinzel pyramidal model through ion channels with optogenetic method. Comput. Methods Programs Biomed. 195, 105665 (2020). https://doi.org/10.1016/j.cmpb.2020.105665
Chen, Y., Ghori, M.B., Kang, Y.: Bifurcation analysis of brain connectivity regulated neural oscillations in schizophrenia. Int. J. Bifurcat. Chaos. 32(11), 2250167 (2022). https://doi.org/10.1142/S021812742250167X
Saturnino, G.B., Madsen, K.H., Thielscher, A.: Electric field simulations for transcranial brain stimulation using FEM: an efficient implementation and error analysis. J. Neural Eng. 16(6), 066032 (2019). https://doi.org/10.1088/1741-2552/ab41ba
Bao, B.C., Liu, Z., Xu, J.P.: Steady periodic memristor oscillator with transient chaotic behaviours. Electron. Lett. 46(3), 228–230 (2010). https://doi.org/10.1049/el.2010.3114
Wu, F., Ma, J., Zhang, G.: A new neuron model under electromagnetic field. Appl. Math. Comput. 347, 590–599 (2019). https://doi.org/10.1016/j.amc.2018.10.087
Wang, C., Tang, J., Ma, J.: Minireview on signal exchange between nonlinear circuits and neurons via field coupling. Eur. Phys. J. Spec. Top. 228, 1907–1924 (2019). https://doi.org/10.1140/epjst/e2019-800193-8
Shampine, L.F., Reichelt, M.W.: The MATLAB ODE suite. SIAM J. Sci. Comput. 18(1), 1–22 (1997). https://doi.org/10.1137/S1064827594276424
Ermentrout, B., Mahajan, A.: Simulating, analyzing, and animating dynamical systems: a guide to XPPAUT for researchers and students. Appl. Mech. Rev. 56(4), 53–53 (2003). https://doi.org/10.1137/1.9780898718195
Kepecs, A., Wang, X.-J.: Analysis of complex bursting in cortical pyramidal neuron models. Neurocomputing 32, 181–187 (2000). https://doi.org/10.1016/S0925-2312(00)00162-4
Harenberg, D., Marelli, S., Sudret, B., Winschel, V.: Uncertainty quantification and global sensitivity analysis for economic models. Quant. Econom. 10(1), 1–41 (2019). https://doi.org/10.3982/QE866
Saltelli, A.: Sensitivity analysis for importance assessment. Risk Anal. 22(3), 579–590 (2002). https://doi.org/10.1111/0272-4332.00040
Sudret, B.: Global sensitivity analysis using polynomial chaos expansions. Reliab. Eng. Syst. Saf. 93(7), 964–979 (2008). https://doi.org/10.1016/j.ress.2007.04.002. (Bayesian Networks in Dependability)
Sobol’, I.M.: Global sensitivity indices for nonlinear mathematical models and their Monte Carlo estimates. Math. Comput. Simul. 55(1), 271–280 (2001). https://doi.org/10.1016/S0378-4754(00)00270-6. (The Second IMACS Seminar on Monte Carlo Methods)
Marelli, S., Sudret, B.: UQLab: A Framework for Uncertainty Quantification in Matlab, pp. 2554–2563 (2014). https://doi.org/10.1061/9780784413609.257
Marino, S., Hogue, I.B., Ray, C.J., Kirschner, D.E.: A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 254(1), 178–196 (2008). https://doi.org/10.1016/j.jtbi.2008.04.011
Longtin, A.: Stochastic resonance in neuron models. J. Stat. Phys. 70(1), 309–327 (1993). https://doi.org/10.1007/BF01053970
Lee, S.-G., Kim, S.: Parameter dependence of stochastic resonance in the stochastic Hodgkin–Huxley neuron. Phys. Rev. E 60(1), 826 (1999). https://doi.org/10.1103/PhysRevE.60.826
Danziger, Z., Grill, W.M.: A neuron model of stochastic resonance using rectangular pulse trains. J. Comput. Neurosci. 38(1), 53–66 (2015). https://doi.org/10.1007/s10827-014-0526-4
Pathmanathan, P., Cordeiro, J.M., Gray, R.A.: Comprehensive uncertainty quantification and sensitivity analysis for cardiac action potential models. Front. Physiol. 10, 721 (2019). https://doi.org/10.3389/fphys.2019.00721
Ghori, M.B., Naik, P.A., Zu, J., Eskandari, Z., Naik, M.: Global dynamics and bifurcation analysis of a fractional-order SEIR epidemic model with saturation incidence rate. Math. Methods. Appl. Sci. 45(7), 3665–3688 (2022). https://doi.org/10.1002/mma.8010
Zi, Z.: Sensitivity analysis approaches applied to systems biology models. IET Syst. Biol. 5, 336–34610 (2011). https://doi.org/10.1049/iet-syb.2011.0015
Lees, J., Jaeger, J.A., Gunn, J.A., Noble, B.F.: Analysis of uncertainty consideration in environmental assessment: an empirical study of Canadian EA practice. J. Environ. Plan. Manag. 59(11), 2024–2044 (2016). https://doi.org/10.1080/09640568.2015.1116980
Bahl, A., Stemmler, M.B., Herz, A.V., Roth, A.: Automated optimization of a reduced layer 5 pyramidal cell model based on experimental data. J. Neurosci. Methods 210(1), 22–34 (2012). https://doi.org/10.1016/j.jneumeth.2012.04.006
Markram, H.: The blue brain project. Nat. Rev. Neurosci. 7(2), 153–160 (2006). https://doi.org/10.1038/nrn1848
Zhu, F., Wang, R., Aihara, K., Pan, X.: Energy-efficient firing patterns with sparse bursts in the Chay neuron model. Nonlinear Dyn. 100(3), 2657–2672 (2020). https://doi.org/10.1007/s11071-020-05593-8
Wang, Y., Xu, X., Wang, R.: Energy features in spontaneous up and down oscillations. Cogn. Neurodyn. 15(1), 65–75 (2021). https://doi.org/10.1007/s11571-020-09597-3
Acknowledgements
The first author acknowledges the Ministry of Education of the People’s Republic of China for its generous support of the China Scholarship Council (CSC) as well as the computational resources provided by the Research Center for Augmented Intelligence at Zhejiang Laboratory in Hangzhou, China.
Funding
This research is financially supported by the National Natural Science Foundation of China (Grant No. 12172268).
Author information
Authors and Affiliations
Contributions
M. B. Ghori proposed the research plan, designed simulations, analyzed results, and drafted the manuscript. Y. Kang supervised and sponsored the research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghori, M.B., Kang, Y. Uncertainty quantification and sensitivity analysis of a hippocampal CA3 pyramidal neuron model under electromagnetic induction. Nonlinear Dyn 111, 13457–13479 (2023). https://doi.org/10.1007/s11071-023-08514-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-023-08514-7