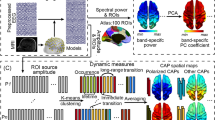
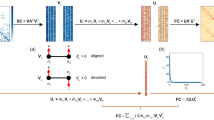
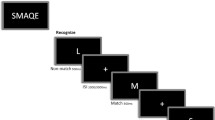
Abstract
The relationship between the age-related reorganization of brain networks and individual behavior has attracted much attention. However, how age induces changes in neural activity at different frequencies in the brain to balance the demands of network integration and segregation, and how age-induced changes in network integration and segregation relate to behavior remain enigmatic. Here, a nested-spectral partition method was used to analyze behavioral-related dynamic functional balance in the aging brain with electroencephalogram signals collected from 56 healthy participants (age: 20–80 years) at rest. The nested-spectral partition approach measures hierarchical segregation and integration across multiple levels by detecting hierarchical modules in brain functional networks. Declines in general personality and general cognitive ability in older adults were captured by exploratory factor analysis. We showed that the brain network of elderly individuals contains more hierarchical modules to generate higher segregation, and it is closer to the functional balance state in the theta and alpha bands but away from this state in the gamma band. Meanwhile, the abnormal variability of functional balance in the elderly brain supports more flexible transitions between segregated and integrated states in the alpha band but reduces the transitions in the beta and gamma bands. Crucially, the degeneration of general personality and general cognitive ability is significantly associated with higher segregation and abnormal flexibility of the brain, especially in the theta, beta, and gamma bands. Our results provide deep insights from a spectral partitioning perspective into the brain dynamic mechanisms that are associated with age-related personality and cognitive degeneration.






Similar content being viewed by others
Data and code availability
Any data and code reported in this paper are available upon request.
References
Soto, C., John, O., Gosling, S., Potter, J.: Age differences in personality traits from 10 to 65: big five domains and facets in a large cross-sectional sample. J. Pers. Soc. Psychol. 100, 330–348 (2011). https://doi.org/10.1037/a0021717
Jiang, R., Calhoun, V.D., Zuo, N., Lin, D., Li, J., Fan, L., Qi, S., Sun, H., Fu, Z., Song, M., Jiang, T., Sui, J.: Connectome-based individualized prediction of temperament trait scores. Neuroimage 183, 366–374 (2018). https://doi.org/10.1016/j.neuroimage.2018.08.038
Yoo, K., Rosenberg, M.D., Hsu, W.-T., Zhang, S., Li, C.-S.R., Scheinost, D., Constable, R.T., Chun, M.M.: Connectome-based predictive modeling of attention: comparing different functional connectivity features and prediction methods across datasets. Neuroimage 167, 11–22 (2018). https://doi.org/10.1016/j.neuroimage.2017.11.010
Beaty, R.E., Kaufman, S.B., Benedek, M., Jung, R.E., Kenett, Y.N., Jauk, E., Neubauer, A.C., Silvia, P.J.: Personality and complex brain networks: the role of openness to experience in default network efficiency. Hum. Brain Mapp. 37, 773–779 (2016). https://doi.org/10.1002/hbm.23065
Langer, N., Pedroni, A., Gianotti, L.R.R., Hänggi, J., Knoch, D., Jäncke, L.: Functional brain network efficiency predicts intelligence. Hum. Brain Mapp. 33, 1393–1406 (2012). https://doi.org/10.1002/hbm.21297
Betzel, R.F., Byrge, L., He, Y., Goñi, J., Zuo, X.-N., Sporns, O.: Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage 102, 345–357 (2014). https://doi.org/10.1016/j.neuroimage.2014.07.067
Damoiseaux, J.S.: Effects of aging on functional and structural brain connectivity. Neuroimage 160, 32–40 (2017). https://doi.org/10.1016/j.neuroimage.2017.01.077
Ferreira, L.K., Busatto, G.F.: Resting-state functional connectivity in normal brain aging. Neurosci. Biobehav. Rev. 37, 384–400 (2013). https://doi.org/10.1016/j.neubiorev.2013.01.017
Wu, J.-T., Wu, H.-Z., Yan, C.-G., Chen, W.-X., Zhang, H.-Y., He, Y., Yang, H.-S.: Aging-related changes in the default mode network and its anti-correlated networks: a resting-state fMRI study. Neurosci. Lett. 504, 62–67 (2011). https://doi.org/10.1016/j.neulet.2011.08.059
Cohen, J.R., D’Esposito, M.: The segregation and integration of distinct brain networks and their relationship to cognition. J. Neurosci. Off. J. Soc. Neurosci. 36, 12083–12094 (2016). https://doi.org/10.1523/JNEUROSCI.2965-15.2016
Fornito, A., Zalesky, A., Breakspear, M.: The connectomics of brain disorders. Nat. Rev. Neurosci. 16, 159–172 (2015). https://doi.org/10.1038/nrn3901
Stam, C.J.: Modern network science of neurological disorders. Nat. Rev. Neurosci. 15, 683–695 (2014). https://doi.org/10.1038/nrn3801
Goh, J.O.S.: Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis. 2, 30–48 (2011)
Wang, R., Lin, P., Liu, M., Wu, Y., Zhou, T., Zhou, C.: Hierarchical connectome modes and critical state jointly maximize human brain functional diversity. Phys. Rev. Lett. 123, 038301 (2019). https://doi.org/10.1103/PhysRevLett.123.038301
Wang, R., Liu, M., Cheng, X., Wu, Y., Hildebrandt, A., Zhou, C.: Segregation, integration, and balance of large-scale resting brain networks configure different cognitive abilities. Proc. Natl. Acad. Sci. 118, e2022288118 (2021). https://doi.org/10.1073/pnas.2022288118
Wang, R., Su, X., Chang, Z., Lin, P., Wu, Y.: Flexible brain transitions between hierarchical network segregation and integration associated with cognitive performance during a multisource interference task. IEEE J. Biomed. Health Inform. 26, 1835–1846 (2022). https://doi.org/10.1109/JBHI.2021.3119940
Babayan, A., Erbey, M., Kumral, D., Reinelt, J.D., Reiter, A.M.F., Röbbig, J., Schaare, H.L., Uhlig, M., Anwander, A., Bazin, P.-L., Horstmann, A., Lampe, L., Nikulin, V.V., Okon-Singer, H., Preusser, S., Pampel, A., Rohr, C.S., Sacher, J., Thöne-Otto, A., Trapp, S., Nierhaus, T., Altmann, D., Arelin, K., Blöchl, M., Bongartz, E., Breig, P., Cesnaite, E., Chen, S., Cozatl, R., Czerwonatis, S., Dambrauskaite, G., Dreyer, M., Enders, J., Engelhardt, M., Fischer, M.M., Forschack, N., Golchert, J., Golz, L., Guran, C.A., Hedrich, S., Hentschel, N., Hoffmann, D.I., Huntenburg, J.M., Jost, R., Kosatschek, A., Kunzendorf, S., Lammers, H., Lauckner, M.E., Mahjoory, K., Kanaan, A.S., Mendes, N., Menger, R., Morino, E., Näthe, K., Neubauer, J., Noyan, H., Oligschläger, S., Panczyszyn-Trzewik, P., Poehlchen, D., Putzke, N., Roski, S., Schaller, M.-C., Schieferbein, A., Schlaak, B., Schmidt, R., Gorgolewski, K.J., Schmidt, H.M., Schrimpf, A., Stasch, S., Voss, M., Wiedemann, A., Margulies, D.S., Gaebler, M., Villringer, A.: A mind-brain-body dataset of MRI, EEG, cognition, emotion, and peripheral physiology in young and old adults. Sci. Data. 6, 180308 (2019). https://doi.org/10.1038/sdata.2018.308
Aydore, S., Pantazis, D., Leahy, R.M.: A note on the phase locking value and its properties. Neuroimage 74, 231–244 (2013). https://doi.org/10.1016/j.neuroimage.2013.02.008
Mahjoory, K., Cesnaite, E., Hohlefeld, F.U., Villringer, A., Nikulin, V.V.: Power and temporal dynamics of alpha oscillations at rest differentiate cognitive performance involving sustained and phasic cognitive control. Neuroimage 188, 135–144 (2019). https://doi.org/10.1016/j.neuroimage.2018.12.001
Zanesco, A.P., King, B.G., Skwara, A.C., Saron, C.D.: Within and between-person correlates of the temporal dynamics of resting EEG microstates. NeuroImage 211, 116631 (2020). https://doi.org/10.1016/j.neuroimage.2020.116631
Aydın, S.: Cross-validated adaboost classification of emotion regulation strategies identified by spectral coherence in resting-state. Neuroinformatics 20, 627–639 (2022). https://doi.org/10.1007/s12021-021-09542-7
Delorme, A., Makeig, S.: EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 134, 9–21 (2004). https://doi.org/10.1016/j.jneumeth.2003.10.009
Xie, Y., Oniga, S.: A review of processing methods and classification algorithm for EEG signal. Carpathian J. Electron. Comput. Eng. 13, 23–29 (2020). https://doi.org/10.2478/cjece-2020-0004
Cohen, J.: A Power Primer. American Psychological Association, Washington, DC, US (2016)
Lachaux, J.-P., Rodriguez, E., Martinerie, J., Varela, F.J.: Measuring phase synchrony in brain signals. Hum. Brain Mapp. 8, 194–208 (1999)
Bakhshayesh, H., Fitzgibbon, S.P., Janani, A.S., Grummett, T.S., Pope, K.J.: Detecting synchrony in EEG: a comparative study of functional connectivity measures. Comput. Biol. Med. 105, 1–15 (2019). https://doi.org/10.1016/j.compbiomed.2018.12.005
Roberts, B.W., Walton, K.E., Viechtbauer, W.: Patterns of mean-level change in personality traits across the life course: a meta-analysis of longitudinal studies. Psychol. Bull. 132, 1–25 (2006). https://doi.org/10.1037/0033-2909.132.1.1
Jones, C.J., Livson, N., Peskin, H.: Longitudinal hierarchical linear modeling analyses of California psychological inventory data from age 33 to 75: an examination of stability and change in adult personality. J. Pers. Assess. 80, 294–308 (2003). https://doi.org/10.1207/S15327752JPA8003_07
Jorm, A.F., Christensen, H., Henderson, A.S., Jacomb, P.A., Korten, A.E., Rodgers, B.: Using the BIS/BAS scales to measure behavioural inhibition and behavioural activation: factor structure, validity and norms in a large community sample. Personal. Individ. Differ. 26, 49–58 (1998). https://doi.org/10.1016/S0191-8869(98)00143-3
Sediyama, C.Y.N., Moura, R., Garcia, M.S., da Silva, A.G., Soraggi, C., Neves, F.S., Albuquerque, M.R., Whiteside, S.P., Malloy-Diniz, L.F.: Factor analysis of the Brazilian version of UPPS impulsive behavior scale. Front. Psychol. 8, 622 (2017)
Geerligs, L., Renken, R.J., Saliasi, E., Maurits, N.M., Lorist, M.M.: A brain-wide study of age-related changes in functional connectivity. Cereb. Cortex N. Y. N 1991(25), 1987–1999 (2015). https://doi.org/10.1093/cercor/bhu012
Ma, J., Lin, Y., Hu, C., Zhang, J., Yi, Y., Dai, Z.: Integrated and segregated frequency architecture of the human brain network. Brain Struct. Funct. 226, 335–350 (2021). https://doi.org/10.1007/s00429-020-02174-8
Fong, A.H.C., Yoo, K., Rosenberg, M.D., Zhang, S., Li, C.-S.R., Scheinost, D., Constable, R.T., Chun, M.M.: Dynamic functional connectivity during task performance and rest predicts individual differences in attention across studies. Neuroimage 188, 14–25 (2019). https://doi.org/10.1016/j.neuroimage.2018.11.057
Deco, G., Jirsa, V.K., McIntosh, A.R.: Resting brains never rest: computational insights into potential cognitive architectures. Trends Neurosci. 36, 268–274 (2013). https://doi.org/10.1016/j.tins.2013.03.001
Pedersen, M., Zalesky, A., Omidvarnia, A., Jackson, G.D.: Multilayer network switching rate predicts brain performance. Proc. Natl. Acad. Sci. 115, 13376–13381 (2018). https://doi.org/10.1073/pnas.1814785115
Malcolm, B.R., Foxe, J.J., Butler, J.S., De Sanctis, P.: The aging brain shows less flexible reallocation of cognitive resources during dual-task walking: a mobile brain/body imaging (MoBI) study. Neuroimage 117, 230–242 (2015). https://doi.org/10.1016/j.neuroimage.2015.05.028
Kabbara, A., Paban, V., Weill, A., Modolo, J., Hassan, M.: Brain network dynamics correlate with personality traits. Brain Connect. 10, 108–120 (2020). https://doi.org/10.1089/brain.2019.0723
Wang, R., Wang, L., Yang, Y., Li, J., Wu, Y., Lin, P.: Random matrix theory for analyzing the brain functional network in attention deficit hyperactivity disorder. Phys. Rev. E 94, 052411 (2016). https://doi.org/10.1103/PhysRevE.94.052411
Wang, R., Zhang, Z.-Z., Ma, J., Yang, Y., Lin, P., Wu, Y.: Spectral properties of the temporal evolution of brain network structure. Chaos Interdiscip. J. Nonlinear Sci. 25, 123112 (2015). https://doi.org/10.1063/1.4937451
Khanna, A., Pascual-Leone, A., Michel, C.M., Farzan, F.: Microstates in resting-state EEG: current status and future directions. Neurosci. Biobehav. Rev. 49, 105–113 (2015). https://doi.org/10.1016/j.neubiorev.2014.12.010
Hilger, K., Fukushima, M., Sporns, O., Fiebach, C.: Temporal stability of functional brain modules associated with human intelligence. Hum. Brain Mapp. 41, 362–372 (2019). https://doi.org/10.1002/hbm.24807
Fiorenzato, E., Strafella, A.P., Kim, J., Schifano, R., Weis, L., Antonini, A., Biundo, R.: Dynamic functional connectivity changes associated with dementia in Parkinson’s disease. Brain 142, 2860–2872 (2019). https://doi.org/10.1093/brain/awz192
Díez-Cirarda, M., Strafella, A.P., Kim, J., Peña, J., Ojeda, N., Cabrera-Zubizarreta, A., Ibarretxe-Bilbao, N.: Dynamic functional connectivity in Parkinson’s disease patients with mild cognitive impairment and normal cognition. NeuroImage Clin. 17, 847–855 (2018). https://doi.org/10.1016/j.nicl.2017.12.013
Faghiri, A., Stephen, J.M., Wang, Y.-P., Wilson, T.W., Calhoun, V.D.: Changing brain connectivity dynamics: from early childhood to adulthood. Hum. Brain Mapp. 39, 1108–1117 (2018). https://doi.org/10.1002/hbm.23896
Geerligs, L., Saliasi, E., Renken, R.J., Maurits, N.M., Lorist, M.M.: Flexible connectivity in the aging brain revealed by task modulations. Hum. Brain Mapp. 35, 3788–3804 (2014). https://doi.org/10.1002/hbm.22437
Duffy, F.H., Albert, M.S., McAnulty, G., Garvey, A.J.: Age-related differences in brain electrical activity of healthy subjects. Ann. Neurol. 16, 430–438 (1984). https://doi.org/10.1002/ana.410160403
Miraglia, F., Vecchio, F., Bramanti, P., Rossini, P.M.: EEG characteristics in “eyes-open” versus “eyes-closed” conditions: small-world network architecture in healthy aging and age-related brain degeneration. Clin. Neurophysiol. 127, 1261–1268 (2016). https://doi.org/10.1016/j.clinph.2015.07.040
Jin, C., Jia, H., Lanka, P., Rangaprakash, D., Li, L., Liu, T., Hu, X., Deshpande, G.: Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Hum. Brain Mapp. 38, 4479–4496 (2017). https://doi.org/10.1002/hbm.23676
Finnigan, S., Robertson, I.H.: Resting EEG theta power correlates with cognitive performance in healthy older adults. Psychophysiology 48, 1083–1087 (2011). https://doi.org/10.1111/j.1469-8986.2010.01173.x
Miltner, W.H.R., Braun, C., Arnold, M., Witte, H., Taub, E.: Coherence of gamma-band EEG activity as a basis for associative learning. Nature 397, 434–436 (1999). https://doi.org/10.1038/17126
Klimesch, W.: EEG-alpha rhythms and memory processes. Int. J. Psychophysiol. 26, 319–340 (1997). https://doi.org/10.1016/S0167-8760(97)00773-3
Lee, K.-H., Williams, L.M., Breakspear, M., Gordon, E.: Synchronous Gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res. Rev. 41, 57–78 (2003). https://doi.org/10.1016/S0165-0173(02)00220-5
Goossens, T., Vercammen, C., Wouters, J., van Wieringen, A.: Aging affects neural synchronization to speech-related acoustic modulations. Front. Aging Neurosci. 8, 133 (2016)
Jaušovec, N., Jaušovec, K.: Personality, gender and brain oscillations. Int. J. Psychophysiol. 66, 215–224 (2007). https://doi.org/10.1016/j.ijpsycho.2007.07.005
Long, N.M., Burke, J.F., Kahana, M.J.: Subsequent memory effect in intracranial and scalp EEG. Neuroimage 84, 488–494 (2014). https://doi.org/10.1016/j.neuroimage.2013.08.052
Sala-Llonch, R., Junqué, C., Arenaza-Urquijo, E.M., Vidal-Piñeiro, D., Valls-Pedret, C., Palacios, E.M., Domènech, S., Salvà, A., Bargalló, N., Bartrés-Faz, D.: Changes in whole-brain functional networks and memory performance in aging. Neurobiol. Aging 35, 2193–2202 (2014). https://doi.org/10.1016/j.neurobiolaging.2014.04.007
Gracia-Tabuenca, Z., Moreno, M.B., Barrios, F.A., Alcauter, S.: Development of the brain functional connectome follows puberty-dependent nonlinear trajectories. NeuroImage 229, 117769 (2021). https://doi.org/10.1016/j.neuroimage.2021.117769
Funding
This work was supported by the National Natural Science Foundation of China (Grants No. 12132012, No. 11972275, No. 12272292, and No. 62071177).
Author information
Authors and Affiliations
Contributions
Conceptualization was contributed by YF; Methodology was contributed by YF and RW; Data curation was contributed by YF; Formal analysis was contributed by YF; Funding acquisition was contributed by RW, Pan Lin and YW; Investigation was contributed by YF, RW and LZ; Software was contributed by YF; Supervision was contributed by RW and YW; Visualization was contributed by YF; Writing—original draft, was contributed by YF; Writing—review & editing, was contributed by YF, RW, PL and YW.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Y., Wang, R., Zhou, L. et al. Nested-spectral analysis reveals a disruption of behavioral-related dynamic functional balance in the aging brain. Nonlinear Dyn 111, 9537–9553 (2023). https://doi.org/10.1007/s11071-023-08328-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-023-08328-7