Abstract
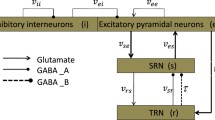
Considering the disinhibition circuit between inhibitory neuronal populations with different time scales in cortical neural networks, here we propose a novel model to describe the occurrences and transitions of epilepsy waveforms. With the model we can successfully simulate poly-spike complexes, which are common in electrophysiological experiments and focal epilepsy patients. Meanwhile, we focus on the dynamic transitions between epilepsy waveforms and normal state and are devoted to exploring effective electrical stimulation strategies. Results show that disinhibition can induce an epileptic bidirectional transition, which is from spike and wave discharges, to poly-spike complexes and then to low-voltage rapid discharge activity, or it is reversed. And fascinating dynamical transition behaviors can be induced by varying average inhibitory synaptic gain. Interestingly, after applying two different control signals (deep brain stimulation and oscillatory input) to the system, all epilepsy waveforms can be suppressed or even eliminated. Results shed light on the pathophysiological mechanisms of epilepsy and guide clinical treatment from a theoretical viewpoint.











Similar content being viewed by others
References
Sauro, K.M., Wiebe, S., Dunkley, C., et al.: The current state of epilepsy guidelines: a systematic review. Epilepsia 57(1), 13–23 (2016)
Paz, J.T., Huguenard, J.R.: Microciruites and their interaction in epilepsy: is the focus out of focus? Nat. Neurosci. 18(3), 351–359 (2015)
Alexander, A., Maroso, M., Soltesz, I.: Organization and control of epileptic circuits in temporal lobe epilepsy. Prog. Brain Res. 226, 127–154 (2016)
Moshé, S.L., Perucca, E., Ryvlin, P., et al.: Epilepsy: new advances. Lancet 385(9971), 884–898 (2015)
Touboul, J., Wendling, F., Chauvel, P., et al.: Neural mass activity, bifurcations, and epilepsy. Neural Comput. 23(12), 3232–3286 (2011)
Fan, X., Gaspard, N., Legros, B., et al.: Dynamics underlying interictal to ICTAL transition in temporal lobe epilepsy: insights from a neural mass model. Eur. J. Neurosci. 47(3), 258–268 (2018)
Wendling, F., Benquet, P., Bartolomei, F., et al.: Computational models of epileptiform activity. J. Neurosci. Methods 260, 233–251 (2016)
Wilson, H.R., Cowan, J.D.: Excitatory and inhibitory interactions in localized populations of model neurons. Biophys. J. 12(1), 1–24 (1972)
Wilson, H.R., Cowan, J.D.: A mathematical theory of the functional dynamics of cortical and thalamic nervous tissue. Kybernetik 13(2), 55–80 (1973)
Da Silva, F.H.L., Hoeks, A., Smits, H., et al.: Model of brain rhythmic activity. Kybernetik 15(1), 27–37 (1974)
Jansen, B.H., Rit, V.G.: Electroencephalogram and visual evoked potential generation in a mathematical model of coupled cortical columns. Biol. Cybern. 73(4), 357–366 (1995)
Wendling, F., Bartolomei, F., Bellanger, J.J., et al.: Epileptic fast activity can be explained by a model of impaired GABAergic dendritic inhibition. Eur. J. Neurosci. 15(9), 1499–1508 (2002)
David, O., Friston, K.J.: A neural mass model for MEG/EEG: coupling and neuronal dynamics. NeuroImage 20(3), 1743–1755 (2003)
Ursino, M., Cona, F., Zavaglia, M.: The generation of rhythms within a cortical region: analysis of a neural mass model. NeuroImage 52(3), 1080–1094 (2010)
Suffczynski, P., Kalitzin, S., Da Silva, F.H.L.: Dynamics of non-convulsive epileptic phenomena modeled by a bistable neuronal network. Neuroscience 126(2), 467–484 (2004)
Grimbert, F., Faugeras, O.: Bifurcation analysis of Jansen’s neural mass model. Neural Comput. 18(12), 3052–3068 (2006)
Wendling, F., Chauvel, P.: Transition to ICTAL activity in temporal lobe epilepsy: insights from macroscopic models. In: Computational Neuroscience in Epilepsy, pp. 356-XIV. Academic Press, Cambridge (2008)
Blenkinsop, A., Valentin, A., Richardson, M.P., et al.: The dynamic evolution of focal-onset epilepsies-combining theoretical and clinical observations. Eur. J. Neurosci. 36(2), 2188–2200 (2012)
Geng, S., Zhou, W.: Influence of extrinsic inputs and synaptic gains on dynamics of Wendling’s neural mass model: a bifurcation analysis. J. Integr. Neurosci. 15(04), 463–483 (2016)
Geng, S., Zhou, W., Zhao, X., et al.: Bifurcation and oscillation in a time-delay neural mass model. Biol. Cybern. 108(6), 747–756 (2014)
Song, J.L., Li, Q., Pan, M., et al.: Seizure tracking of epileptic EEGs using a model-driven approach. J. Neural Eng. 17(1), 016–024 (2020)
Wendling, F., Bellanger, J.J., Bartolomei, F., et al.: Relevance of nonlinear lumped-parameter models in the analysis of depth-EEG epileptic signals. Biol. Cybern. 83(4), 367–378 (2000)
Cao, Y., Ren, K., Su, F., et al.: Suppression of seizures based on the multi-coupled neural mass model. Chaos Interdiscip. J. Nonlinear Sci. 25(10), 103–120 (2015)
Ahmadizadeh, S., Karoly, P.J., Nešić, D., et al.: Bifurcation analysis of two coupled Jansen-Rit neural mass models. PloS ONE 13(3), e0192842 (2018)
Liang, S., Wang, Z.: Controlling a neuron by stimulating a coupled neuron. Appl. Math. Mech. 40(1), 13–24 (2019)
Ge, Y., Cao, Y., Yi, G., et al.: Robust closed-loop control of spike-and-wave discharges in a thalamocortical computational model of absence epilepsy. Sci. Rep. 9(1), 1–16 (2019)
Wang, Z., Wang, Q.: Stimulation strategies for absence seizures: targeted therapy of the focus in coupled thalamocortical model. Nonlinear Dyn. 96(2), 1649–1663 (2019)
Li, J., Liu, S., Liu, W., Yu, Y., Wu, Y.: Suppression of firing activities in neuron and neurons of network induced by electromagnetic radiation. Nonlinear Dyn. 83(1–2), 801–810 (2016)
Lv, M., Ma, J.: Model of electrical activity in a neuron under magnetic flow. Nonlinear Dyn. 85, 1479–1490 (2016)
Fan, D., Wang, Q., Perc, M.: Disinhibition-induced transitions between absence and tonic-clonic epileptic seizures. Sci. Rep. 5, 12618 (2015)
Fan, D., Liu, S., Wang, Q.: Stimulus-induced epileptic spike-wave discharges in thalamocortical model with disinhibition. Sci. Rep. 6(1), 1–21 (2016)
Liu, S., Wang, Q.: Transition dynamics of generalized multiple epileptic seizures associated with thalamic reticular nucleus excitability: a computational study. Commun. Nonlinear Sci. Numer. Simul. 52, 203–213 (2017)
Liu, S., Wang, Q., Fan, D.: Disinhibition-induced delayed onset of epileptic spike-wave discharges in a five variable model of cortex and thalamus. Front. Comput. Neurosci. 10, 28 (2016)
Pi, H.J., Hangya, B., Kvitsiani, D., et al.: Cortical interneurons that specialize in disinhibitory control. Nature 503(7477), 521–524 (2013)
Chen, M., Guo, D., et al.: Critical roles of the direct GABAergic Pallido-cortical pathway in controlling absence seizures. PLoS Comput. Biol. 11(10), e1004539 (2015)
Liu, F., Wang, J., Liu, C., et al.: A neural mass model of basal ganglia nuclei simulates pathological beta rhythm in Parkinson’s disease. Chaos 26(12), 123113 (2016)
Bettus, G., Wendling, F., Guye, M., et al.: Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 81(1), 58–68 (2008)
Noebels, J.L.: Targeting epilepsy genes. Neuron 16(2), 241–244 (1996)
Muñoz, A., Méndez, P., DeFelipe, J., et al.: Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus. Epilepsia 48(4), 663–673 (2007)
Haghighi, H.S., Markazi, A.H.D.: Dynamic origin of spike and wave discharges in the brain. Neuroimage 197, 69–79 (2019)
Marten, F., Rodrigues, S., Benjamin, O., et al.: Onset of polyspike complexes in a mean-field model of human electroencephalography and its application to absence epilepsy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 367(1891), 1145–1161 (2009)
Marten, F., Rodrigues, S., Suffczynski, P., et al.: Derivation and analysis of an ordinary differential equation mean-field model for studying clinically recorded epilepsy dynamics. Phys. Rev. E 79(2), 021911 (2009)
Hebbink, G.J.: Activity Types in a Neural Mass Model. University of Twente, Enschede (2014)
Dodt, H.U., Pawelzik, H., Zieglga, W.: Actions of noradrenaline on neocortical neurons in vitro. Brain Res. 545(1–2), 307–311 (1991)
Van Rotterdam, A., Da Silva, F.H.L., Van den Ende, J., et al.: A model of the spatial-temporal characteristics of the alpha rhythm. Bull. Math. Biol. 44(2), 283–305 (1982)
Arrais, M., Wendling, F., Modolo, J.: Identification of effective stimulation parameters to abort epileptic seizures in a neural mass model. In: 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, pp. 5208–5211 (2019)
Abbott, L.F.: Lapicque’s introduction of the integrate-and-fire model neuron. Brain Res. Bull. 50(5–6), 303–304 (1907)
Citri, A., Malenka, R.C.: Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33(1), 18–41 (2008)
Kile, K.B., Tian, N., Durand, D.M.: Low frequency stimulation decreases seizure activity in a mutation model of epilepsy. Epilepsia 51(9), 1745–1753 (2010)
Bikson, M., Lian, J., Hahn, P.J., et al.: Suppression of epileptiform activity by high frequency sinusoidal fields in rat hippocampal slices. J. Physiol. 531(Pt 1), 181 (2001)
Chiang, C.C., Lin, C.C.K., Ju, M.S., et al.: High frequency stimulation can suppress globally seizures induced by 4-AP in the rat hippocampus: an acute in vivo study. Brain Stimul. 6(2), 180–189 (2013)
Boon, P., Vonck, K., De Herdt, V., et al.: Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia 48(8), 1551–1560 (2007)
Fisher, R.S., Velasco, A.L.: Electrical brain stimulation for epilepsy. Nat. Rev. Neurol. 10(5), 261–270 (2014)
Zhang, H., Wang, Q., Chen, G.: Control effects of stimulus paradigms on characteristic firings of parkinsonism. Chaos 24(3), 033134 (2014)
Wichmann, T., Soares, J.: Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J. Neurophysiol. 95(4), 2120–2133 (2006)
Rivlin-Etzion, M., Marmor, O., Saban, G., et al.: Low-Pass filter properties of basal ganglia-cortical-muscle loops in the normal and MPTP primate model of Parkinsonism. J. Neurosci. 28(3), 633–649 (2008)
Wang, Z., Wang, Q.: Eliminating absence seizures through the deep brain stimulation to thalamus reticular nucleus. Front. Comput. Neurosci. 11 (2017)
Hu, B., Wang, Q.: Controlling absence seizures by deep brain stimulus applied on substantia nigra pars reticulata and cortex. Chaos Solitons Fractals Appl. Sci. Eng. Interdiscipl. J. Nonlinear Sci. 80, 13–23 (2015)
Zhang, H., Deng, Z., Liu, S.: Transition dynamics of generalized periodic discharges observed in EEG waveforms. Int. J. Bifurcat. Chaos 29(13), 1930038 (2019)
Hu, B., Guo, Y., Zou, X., et al.: Controlling mechanism of absence seizures by deep brain stimulus applied on subthalamic nucleus. Cognit. Neurodyn. 12, 103–119 (2018)
Chen, M., Guo, D., et al.: Bidirectional control of absence seizures by the basal ganglia: a computational evidence. PLoS Comput. Biol. 10(3), e1003495 (2014)
Li, J., Tang, J., Ma, J., Du, M., Wang, R., Wu, Y.: Dynamic transition of neuronal firing induced by abnormal astrocytic glutamate oscillation. Sci. Rep. 6, 32343 (2016)
Mondal, A., Upadhyay, R.K., Ma, J., et al.: Bifurcation analysis and diverse firing activities of a modified excitable neuron model. Cognit. Neurodyn. 13(4), 393–407 (2019)
Zhang, T., Pan, X., Xu, X., Wang, R.B.: A cortical model with multi-layers to study visual attentional modulation of neurons at the synaptic level. Cognit. Neurodyn. 13(6), 579–599 (2019)
Bera, B.K., Ghosh, D., Banerjee, T.: Imperfect traveling chimera states induced by local synaptic gradient coupling. Phys. Rev. E 94(1), 012215 (2016)
Sarbendu, R., Arnob, R., Bera, B.K., et al.: Synchronization and firing patterns of coupled Rulkov neuronal map. Nonlinear Dyn. 94, 785–805 (2018)
Soumen, M., Bidesh, K.B., Dibakar, G., et al.: Chimera states in neuronal networks: a review. Phys. Life Rev. 28, 100–121 (2019)
Bidesh, K., Sarbendu, R., Dibakar, G., et al.: Spike chimera states and firing regularities in neuronal hypernetworks. Chaos 29, 053115 (2019)
Acknowledgements
The authors thank the reviewer for their careful reading and suggestions. The research was supported by the National Natural Science Foundation of China (Nos. 11872304, 11972292) and Postdoctoral Research Foundation of China (No. 2017M623233).
Author information
Authors and Affiliations
Contributions
ZS and HZ conceived of the presented idea. ZS developed the theory, performed the numerical modelings and took the lead in writing the manuscript. HZ provided modeling ideas. ZD gave innovation guidance. LD, LY and PX helped to verify the results and give constructive suggestions. All authors discussed the results and contributed to this research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, Z., Deng, Z., Du, L. et al. Control and analysis of epilepsy waveforms in a disinhibition model of cortex network. Nonlinear Dyn 103, 2063–2079 (2021). https://doi.org/10.1007/s11071-020-06131-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-020-06131-2