Abstract
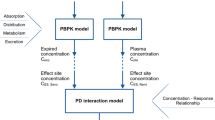
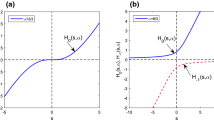
This paper discusses a possibility to simplify the number of parameters in the Hill curve by exploiting special mathematical functions. This simplification is relevant when adaptation is required for personalized model-based medicine during continuous monitoring of dose–response values. A mathematical framework of the involved physiology and modelling by means of distributed parameter progressions has been employed. Convergence to a unique dynamic response is achieved, allowing simplifying assumptions with guaranteed solution. Discussion on its use and comparison with other adaptation mechanism is provided.




Similar content being viewed by others
References
Neckebroek, M., De Smet, T., Struys, M.: Automated drug delivery in anesthesia. Curr. Anesthesiol. Rep. 3, 18–26 (2013)
Ionescu, C., De Keyser, R., Claure-Torrico, B., De Smet, T., Struys, M., Normey-Rico, J.: Robust predictive control strategy applied for propofol using BIS as a controlled variable during anesthesias. IEEE Trans. Biomed. Eng. 55(9), 2161–2170 (2008)
Ionescu, C., Machado, J., De Keyser, R., Decruyenaere, J., Struys, M.: Nonlinear dynamics of the patient’s response to drug effect during general anesthesia. Commun. Nonlinear Sci. Numer. Simul. 20(3), 914–926 (2015)
Nino, J., De Keyser, R., Syafiie, S., Ionescu, C., Struys, M.: EPSAC controlled anesthesia with online gain adaptation, in special issue “Trust me I am a doctor” of the Int. J. Adapt. Control Signal Process. 23, 455–471 (2009)
Goutelle, S., Maurin, M., Rougier, F., Barbaut, X., Bourguignon, L., Ducher, M., Maire, P.: The Hill equation: a review of its capabilities in pharmacological modelling. Fundam. Clin. Pharmacol. 22, 633–648 (2008)
Rinaki, E., Valsami, G., Macheras, P.: The power-law can describe the entire drug release curve from HPMC-based matrix tablets: a hypothesis. Int. J. Pharm. 255, 199–207 (2003)
Drew, P., Abbott, L.: Models and properties of power-law adaptation in neural systems. J. Neurophysiol. 96, 826–833 (2006)
Weiss, J.: The Hill equation revisited: uses and misuses. FASEB J. 11, 835–841 (1997)
De Keyser, R., Ionescu, C.: A no-nonsense control engineering approach to anaesthesia control during induction phase. In: (ed.) IFAC 8th Symposium on Biological and Medical Systems, Budapest, Hungary, 29–31 August. IFAC, pp. 379–384 (2012)
Mendonca, T., Lemos, J., Magalhaes, H., Rocha, P., Esteves, S.: Drug delivery for neuromuscular blockade with supervised multimodel adaptive control. IEEE Trans. Control Syst. Technol. 17, 1237–1244 (2009)
Padula, F., Ionescu, C., Latronico, N., Paltenghi, M., Visioli, A., Vivacqua, G.: Inversion-based propofol dosing for intravenous induction of hypnosis. Commun. Nonlinear Sci. Numer. Simul. 39, 481–494 (2016)
Padula, F., Ionescu, C., Latronico, N., Paltenghi, M., Visioli, A., Vivacqua, G.: Optimized PID control of depth of hypnosis in anesthesia. Comput. Methods Programs Biomed. 144, 21–35 (2017)
Struys, M.M.R.F., De Smet, T., Glen, J.B., Vereecke, H.E.M., Absalom, A.R., Schnider, T.W.: The history of target-controlled infusion. Anesth. Analg. 122, 56–69 (2016)
De Smet, T., Struys, M.M.R.F., Greenwald, S., Mortier, E.P., Shafer, S.L.: Estimation of optimal modeling weights for a bayesian-based closed-loop system for propofol administration using the bispectral index as a controlled variable: a simulation study. Anesth. Analg. 105, 1629–1638 (2007)
Dokoumetzidis, A., Macheras, P.: Fractional kinetics in drug absorption and disposition processes. J. Pharmacokinet. Biopharm. 36, 165–178 (2009)
Pereira, L.: Fractal pharmacokinetics. Comput. Math. Methods Med. 11(2), 161–184 (2010)
Butanda, J., Malaga, C., Plaza, G.: On the stabilizing effect of chemotaxis on bacterial aggregation patterns. Appl. Math. Nonlinear Sci. 2(1), 157–172 (2017)
Soltesz, K., Hahn, J., Hägglund, T., Dumont, G., Ansermino, J.: Individualized closed-loop control of propofol anesthesia: a preliminary study. Biomed. Signal Process. Control 8(6), 500–508 (2013)
Shieh, J., Linkens, D., Asbury, A.: A hierarchical system of online advisory for monitoring and controlling the depth of anesthesia using self-organizing fuzzy logic. Eng. Appl. Artif. Intell. 18(3), 307–316 (2005)
Ionescu, C., Nascu, I., De Keyser, R.: Lessons learned from closed loops in engineering: towards a multivariable approach regulating depth of anaesthesia. J. Clin. Monit. Comput. 28(6), 537–546 (2014)
Perez-Garcia, V., Fitzpatrick, S., Perez-Romasanta, L., Pesic, M., Schucht, P., Arana, E., Sanchez-Gomez, P.: Applied mathematics and nonlinear sciences in the war on cancer. App. Math. Nonlinear Sci. 1(2), 423–436 (2016)
Ionescu, C., Copot, D., De Keyser, R.: Modelling doxorubicin effect in various cancer therapies by means of fractional calculus, In: (ed.) American Control Conference, Boston, USA. IEEE, pp. 1283–1288 (2016)
Ionescu, C., Copot, D., De Keyser, R.: Anesthesiologist in the loop and predictive algorithm to maintain hypnosis while mimicking surgical disturbance, In: (ed.) IFAC 20th World Congress, Toulouse, France, 9–14 July. IFAC, pp. 15080–15085 (2017)
Weiss, M.: Comparison of distributed and compartmental models of drug disposition: assessment of tissue uptake kinetics. J. Pharmacokinet. Pharmacodyn. 43, 505–512 (2016)
Luginbuhl, M., Gerber, A., Schnider, T., Petersen-Felix, S., Arendt-Nielsen, L., Curatolo, M.: Modulation of remifentanil-induced analgesia, hyperalgesia and tolerance by small-dose ketamine in humans. Anesth. Analg. 96, 726–732 (2003)
Copot, D., Magin, R., De Keyser, R., Ionescu, C.: Data-driven modelling of drug tissue trapping using anomalous kinetics. Chaos Solitons Fractals Online First (2017). https://doi.org/10.1016/j.chaos.2017.03.031
Acknowledgements
C. M. Ionescu is a postdoctoral fellow of the Flanders Research Centre (FWO), Grant No. 12B3415N. This research is financially supported by Flanders Research Centre, Grant No. G026514N.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ionescu, C.M. A computationally efficient Hill curve adaptation strategy during continuous monitoring of dose–effect relation in anaesthesia. Nonlinear Dyn 92, 843–852 (2018). https://doi.org/10.1007/s11071-018-4095-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-018-4095-3