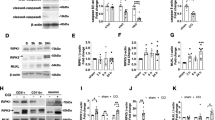
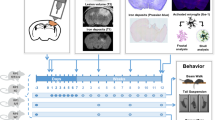
Abstract
Traumatic brain injury (TBI) is a global public safety issue that poses a threat to death, characterized by high fatality rates, severe injuries and low recovery rates. There is growing evidence that necroptosis regulates the pathophysiological processes of a variety of diseases, particularly those affecting the central nervous system. Thus, moderate necroptosis inhibition may be helpful in the management of TBI. Receptor-interacting protein kinase (RIP) 3 is a key mediator in the necroptosis, and its absence helps restore the microenvironment at the injured site and improve cognitive impairment after TBI. In this report, we review different domains of RIP3, multiple analyses of necroptosis, and associations between necroptosis and TBI, RIP3, RIP1, and mixed lineage kinase domain-like. Next, we elucidate the potential involvement of RIP3 in TBI and highlight how RIP3 deficiency enhances neuronal function.


Similar content being viewed by others
Data Availability
Not applicable.
References
TBI-related deaths (2019) Centers for Disease Control and Prevention, https://www.cdc.gov/traumaticbraininjury/data/index.html. Accessed 15 April 2020,
GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators (2019) Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Neurol 18(1):56–87. https://doi.org/10.1016/S1474-4422(18)30415-0
Lee HF, Chen CH, Chang CF (2020) A preclinical controlled cortical impact model for traumatic hemorrhage contusion and neuroinflammation. J Vis Exp 16010.3791/61393
Omer M, Posti JP, Gissler M, Merikukka M, Bärnighausen T, Wilson ML (2022) Birth order and pediatric traumatic brain injury. Sci Rep 12(1):14451. https://doi.org/10.1038/s41598-022-18742-3
Narouiepour A, Ebrahimzadeh-Bideskan A, Rajabzadeh G, Gorji A, Negah SS (2022) Neural stem cell therapy in conjunction with curcumin loaded in niosomal nanoparticles enhanced recovery from traumatic brain injury. Sci Rep 12(1):3572. https://doi.org/10.1038/s41598-022-07367-1
Hegdekar N, Lipinski MM, Sarkar C (2021) N-Acetyl-L-leucine improves functional recovery and attenuates cortical cell death and neuroinflammation after traumatic brain injury in mice. Sci Rep 11(1):9249. https://doi.org/10.1038/s41598-021-88693-8
Nikolian VC, Dekker SE, Bambakidis T, Higgins GA, Dennahy IS, Georgoff PE, Williams AM, Andjelkovic AV, Alam HB (2018) Improvement of blood-brain Barrier Integrity in Traumatic Brain Injury and hemorrhagic shock following treatment with Valproic Acid and Fresh Frozen plasma. Crit Care Med 46(1):e59–e66. https://doi.org/10.1097/CCM.0000000000002800
Jahanbazi Jahan-Abad A, Sahab Negah S, Hosseini Ravandi H, Ghasemi S, Borhani-Haghighi M, Stummer W, Gorji A, Khaleghi Ghadiri M (2018) Human neural Stem/Progenitor cells derived from epileptic human brain in a self-assembling peptide Nanoscaffold Improve Traumatic Brain Injury in rats. Mol Neurobiol 55(12):9122–9138. https://doi.org/10.1007/s12035-018-1050-8
Luo ML, Pan L, Wang L, Wang HY, Li S, Long ZY, Zeng L, Liu Y (2019) Transplantation of NSCs promotes the recovery of cognitive functions by regulating neurotransmitters in rats with traumatic brain Injury. Neurochem Res 44(12):2765–2775. https://doi.org/10.1007/s11064-019-02897-z
Zhang Y, Zhang Y, Chopp M, Zhang ZG, Mahmood A, Xiong Y (2020) Mesenchymal stem cell-derived Exosomes improve functional recovery in rats after traumatic Brain Injury: a dose-response and therapeutic window study. Neurorehabil Neural Repair 34(7):616–626. https://doi.org/10.1177/1545968320926164
Lin SJ, Cao LX, Cheng SB, Dai QF, Lin JH, Pu L, Chen WH, Zhang YJ, Chen SL, Zhang YM (2018) Effect of acupuncture on the TLR2/4-NF-κB signalling pathway in a rat model of traumatic brain injury. Acupunct Med 36(4):247–253. https://doi.org/10.1136/acupmed-2017-011472
Begemann M, Leon M, van der Horn HJ, van der Naalt J, Sommer I (2020) Drugs with anti-inflammatory effects to improve outcome of traumatic brain injury: a meta-analysis. Sci Rep 10(1):16179. https://doi.org/10.1038/s41598-020-73227-5
Oliveira SR, Dionísio PA, Brito H, Franco L, Rodrigues CAB, Guedes RC, Afonso CAM, Amaral JD, Rodrigues CMP (2018) Phenotypic screening identifies a new oxazolone inhibitor of necroptosis and neuroinflammation. Cell Death Discov 4:10. https://doi.org/10.1038/s41420-018-0067-0
He S, Wang X (2018) RIP kinases as modulators of inflammation and immunity. Nat Immunol 19(9):912–922. https://doi.org/10.1038/s41590-018-0188-x
Liu Y, Liu T, Lei T, Zhang D, Du S, Girani L, Qi D, Lin C, Tong R, Wang Y (2019) RIP1/RIP3-regulated necroptosis as a target for multifaceted disease therapy (review). Int J Mol Med 44(3):771–786. https://doi.org/10.3892/ijmm.2019.4244
Khan N, Lawlor KE, Murphy JM, Vince JE (2014) More to life than death: molecular determinants of necroptotic and non-necroptotic RIP3 kinase signaling. Curr Opin Immunol 26:76–89. https://doi.org/10.1016/j.coi.2013.10.017
Jayakumar A, Bothwell ALM (2019) RIPK3-Induced inflammation by I-MDSCs promotes intestinal tumors. Cancer Res 79(7):1587–1599. https://doi.org/10.1158/0008-5472.CAN-18-2153
Liu ZM, Chen QX, Chen ZB, Tian DF, Li MC, Wang JM, Wang L, Liu BH, Zhang SQ, Li F, Ye H, Zhou L (2018) RIP3 deficiency protects against traumatic brain injury (TBI) through suppressing oxidative stress, inflammation and apoptosis: dependent on AMPK pathway. Biochem Biophys Res Commun 499(2):112–119. https://doi.org/10.1016/j.bbrc.2018.02.150
Zhang Y, Li M, Li X, Zhang H, Wang L, Wu X, Zhang H, Luo Y (2020) Catalytically inactive RIP1 and RIP3 deficiency protect against acute ischemic stroke by inhibiting necroptosis and neuroinflammation. Cell Death Dis 11(7):565. https://doi.org/10.1038/s41419-020-02770-w
Guo C, Fu R, Zhou M, Wang S, Huang Y, Hu H, Zhao J, Gaskin F, Yang N, Fu SM (2019) Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J Autoimmun 103:102286. https://doi.org/10.1016/j.jaut.2019.05.014
Ma F, Zhu Y, Chang L, Gong J, Luo Y, Dai J, Lu H (2022) Hydrogen sulfide protects against ischemic heart failure by inhibiting RIP1/RIP3/MLKL-mediated necroptosis. Physiol Res 71(6):771–781. https://doi.org/10.33549/physiolres.934905
Duan C, Xu X, Lu X, Wang L, Lu Z (2022) RIP3 knockdown inhibits necroptosis of human intestinal epithelial cells via TLR4/MyD88/NF-κB signaling and ameliorates murine colitis. BMC Gastroenterol 22(1):137. https://doi.org/10.1186/s12876-022-02208-x
Dionísio PA, Oliveira SR, Gaspar MM, Gama MJ, Castro-Caldas M, Amaral JD, Rodrigues CMP (2019) Ablation of RIP3 protects from dopaminergic neurodegeneration in experimental Parkinson’s disease. Cell Death Dis 10(11):840. https://doi.org/10.1038/s41419-019-2078-z
Wang Y, Song M, Zhou P, Wang J, Zheng J, Xu H (2021) TNFAIP3-upregulated RIP3 exacerbates acute pancreatitis via activating NLRP3 inflammasome. Int Immunopharmacol 100:108067. https://doi.org/10.1016/j.intimp.2021.108067
Chen D, Gregory AD, Li X, Wei J, Burton CL, Gibson G, Scott SJ, St Croix CM, Zhang Y, Shapiro SD (2021) RIP3-dependent necroptosis contributes to the pathogenesis of chronic obstructive pulmonary disease. JCI Insight 6(12):e144689. https://doi.org/10.1172/jci.insight.144689
Wei S, Zhou H, Wang Q, Zhou S, Li C, Liu R, Qiu J, Shi C, Lu L (2019) RIP3 deficiency alleviates liver fibrosis by inhibiting ROCK1-TLR4-NF-κB pathway in macrophages. FASEB J 33(10):11180–11193. https://doi.org/10.1096/fj.201900752R
Ma D, Wang X, Liu X, Li Z, Liu J, Cao J, Wang G, Guo Y, Zhao S (2022) Macrophage infiltration initiates RIP3/MLKL-Dependent necroptosis in Paclitaxel-Induced Neuropathic Pain. Mediators Inflamm 2022:1567210. https://doi.org/10.1155/2022/1567210
Liu S, Joshi K, Denning MF, Zhang J (2021) RIPK3 signaling and its role in the pathogenesis of cancers. Cell Mol Life Sci 78(23):7199–7217. https://doi.org/10.1007/s00018-021-03947-y
Zhang H, Wu X, Li X, Li M, Li F, Wang L, Zhang X, Zhang Y, Luo Y, Wang H, Jiang Y, Zhang H (2020) Crucial roles of the RIP homotypic Interaction Motifs of RIPK3 in RIPK1-Dependent cell death and lymphoproliferative disease. Cell Rep 31(7):107650. https://doi.org/10.1016/j.celrep.2020.107650
Weber K, Roelandt R, Bruggeman I, Estornes Y, Vandenabeele P (2018) Nuclear RIPK3 and MLKL contribute to cytosolic necrosome formation and necroptosis. Commun Biol 1:6. https://doi.org/10.1038/s42003-017-0007-1
Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC (2000) The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett 473(3):285–291. https://doi.org/10.1016/s0014-5793(00)01473-3
Hanna-Addams S, Liu S, Liu H, Chen S, Wang Z, CK1α (2020) CK1δ, and CK1ε are necrosome components which phosphorylate serine 227 of human RIPK3 to activate necroptosis. Proc Natl Acad Sci U S A 117(4):1962–1970. https://doi.org/10.1073/pnas.1917112117
Choi SW, Park HH, Kim S, Chung JM, Noh HJ, Kim SK, Song HK, Lee CW, Morgan MJ, Kang HC, Kim YS (2018) PELI1 selectively targets kinase-active RIP3 for Ubiquitylation-Dependent Proteasomal Degradation. Mol Cell 70(5):920–935e7. https://doi.org/10.1016/j.molcel.2018.05.016
Lim J, Park H, Heisler J, Maculins T, Roose-Girma M, Xu M, Mckenzie B, van Lookeren Campagne M, Newton K, Murthy A (2019) Autophagy regulates inflammatory programmed cell death via turnover of RHIM-domain proteins. Elife 8:e44452. https://doi.org/10.7554/eLife.44452
Muendlein HI, Connolly WM, Magri Z, Smirnova I, Ilyukha V, Gautam A, Degterev A, Poltorak A (2021) ZBP1 promotes LPS-induced cell death and IL-1β release via RHIM-mediated interactions with RIPK1. Nat Commun 12(1):86. https://doi.org/10.1038/s41467-020-20357-z
Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM (2002) Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem 277(11):9505–9511. https://doi.org/10.1074/jbc.M109488200
Mei P, Xie F, Pan J, Wang S, Gao W, Ge R, Gao B, Gao S, Chen X, Wang Y, Wu J, Ding C, Li J (2021) E3 ligase TRIM25 ubiquitinates RIP3 to inhibit TNF induced cell necrosis. Cell Death Differ 28(10):2888–2899. https://doi.org/10.1038/s41418-021-00790-3
Pazdernik NJ, Donner DB, Goebl MG, Harrington MA (1999) Mouse receptor interacting protein 3 does not contain a caspase-recruiting or a death domain but induces apoptosis and activates NF-kappaB. Mol Cell Biol 19(10):6500–6508. https://doi.org/10.1128/MCB.19.10.6500
Li R, Zhao X, Zhang S, Dong W, Zhang L, Chen Y, Li Z, Yang H, Huang Y, Xie Z, Wang W, Li C, Ye Z, Dong Z, Liang X (2021) RIP3 impedes transcription factor EB to suppress autophagic degradation in septic acute kidney injury. Cell Death Dis 12(6):593. https://doi.org/10.1038/s41419-021-03865-8
Zeng X, Zhang YD, Ma RY, Chen YJ, Xiang XM, Hou DY, Li XH, Huang H, Li T, Duan CY (2022) Activated Drp1 regulates p62-mediated autophagic flux and aggravates inflammation in cerebral ischemia-reperfusion via the ROS-RIP1/RIP3-exosome axis. Mil Med Res 9(1):25. https://doi.org/10.1186/s40779-022-00383-2
Lamade AM, Wu L, Dar HH, Mentrup HL, Shrivastava IH, Epperly MW, St Croix CM, Tyurina YY, Anthonymuthu TS, Yang Q, Kapralov AA, Huang Z, Mao G, Amoscato AA, Hier ZE, Artyukhova MA, Shurin G, Rosenbaum JC, Gough PJ, Bertin J, VanDemark AP, Watkins SC, Mollen KP, Bahar I, Greenberger JS, Kagan VE, Whalen MJ, Bayır H (2022) Inactivation of RIP3 kinase sensitizes to 15LOX/PEBP1-mediated ferroptotic death. Redox Biol 50:102232. https://doi.org/10.1016/j.redox.2022.102232
Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM (1999) RIP3, a novel apoptosis-inducing kinase. J Biol Chem 274(24):16871–16875. https://doi.org/10.1074/jbc.274.24.16871
Meng Y, Horne CR, Samson AL, Dagley LF, Young SN, Sandow JJ, Czabotar PE, Murphy JM (2022) Human RIPK3 C-lobe phosphorylation is essential for necroptotic signaling. Cell Death Dis 13(6):565. https://doi.org/10.1038/s41419-022-05009-y
Wu X, Ma Y, Zhao K, Zhang J, Sun Y, Li Y, Dong X, Hu H, Liu J, Wang J, Zhang X, Li B, Wang H, Li D, Sun B, Lu J, Liu C (2021) The structure of a minimum amyloid fibril core formed by necroptosis-mediating RHIM of human RIPK3. Proc Natl Acad Sci U S A 118(14):e2022933118. https://doi.org/10.1073/pnas.2022933118
Riebeling T, Kunzendorf U, Krautwald S (2022) The role of RHIM in necroptosis. Biochem Soc Trans 50(4):1197–1205. https://doi.org/10.1042/BST20220535
Hu H, Wu X, Wu G, Nan N, Zhang J, Zhu X, Zhang Y, Shu Z, Liu J, Liu X, Lu J, Wang H (2021) RIP3-mediated necroptosis is regulated by inter-filament assembly of RIP homotypic interaction motif. Cell Death Differ 28(1):251–266. https://doi.org/10.1038/s41418-020-0598-9
Arrázola MS, Lira M, Véliz-Valverde F, Quiroz G, Iqbal S, Eaton SL, Lamont DJ, Huerta H, Ureta G, Bernales S, Cárdenas JC, Cerpa W, Wishart TM, Court FA (2023) Necroptosis inhibition counteracts neurodegeneration, memory decline, and key hallmarks of aging, promoting brain rejuvenation. Aging Cell 22(5):e13814. https://doi.org/10.1111/acel.13814
Wehn AC, Khalin I, Duering M, Hellal F, Culmsee C, Vandenabeele P, Plesnila N, Terpolilli NA (2021) RIPK1 or RIPK3 deletion prevents progressive neuronal cell death and improves memory function after traumatic brain injury. Acta Neuropathol Commun 9(1):138. https://doi.org/10.1186/s40478-021-01236-0
Zhao P, Wei Y, Sun G, Xu L, Wang T, Tian Y, Chao H, Tu Y, Ji J (2022) Fetuin-A alleviates neuroinflammation against traumatic brain injury-induced microglial necroptosis by regulating Nrf-2/HO-1 pathway. J Neuroinflammation 19(1):269. https://doi.org/10.1186/s12974-022-02633-5
Bao Z, Fan L, Zhao L, Xu X, Liu Y, Chao H, Liu N, You Y, Liu Y, Wang X, Ji J (2019) Silencing of A20 aggravates neuronal death and inflammation after traumatic Brain Injury: a potential trigger of necroptosis. Front Mol Neurosci 12:222. https://doi.org/10.3389/fnmol.2019.00222
Morgan MJ, Kim YS (2022) Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp Mol Med 54(10):1695–1704. https://doi.org/10.1038/s12276-022-00868-z
Füllsack S, Rosenthal A, Wajant H, Siegmund D (2019) Redundant and receptor-specific activities of TRADD, RIPK1 and FADD in death receptor signaling. Cell Death Dis 10(2):122. https://doi.org/10.1038/s41419-019-1396-5
Horne CR, Samson AL, Murphy JM (2023) The web of death: the expanding complexity of necroptotic signaling. Trends Cell Biol 33(2):162–174. https://doi.org/10.1016/j.tcb.2022.05.008
Yu Z, Jiang N, Su W, Zhuo Y, Necroptosis (2021) A novel pathway in Neuroinflammation. Front Pharmacol 12:701564. https://doi.org/10.3389/fphar.2021.701564
Ju E, Park KA, Shen HM, Hur GM (2022) The resurrection of RIP kinase 1 as an early cell death checkpoint regulator-a potential target for therapy in the necroptosis era. Exp Mol Med 54(9):1401–1411. https://doi.org/10.1038/s12276-022-00847-4
Mompeán M, Li W, Li J, Laage S, Siemer AB, Bozkurt G, Wu H, McDermott AE (2018) The structure of the Necrosome RIPK1-RIPK3 core, a human hetero-amyloid signaling complex. Cell 173(5):1244–1253e10. https://doi.org/10.1016/j.cell.2018.03.032
Meng H, Liu Z, Li X, Wang H, Jin T, Wu G, Shan B, Christofferson DE, Qi C, Yu Q, Li Y, Yuan J (2018) Death-domain dimerization-mediated activation of RIPK1 controls necroptosis and RIPK1-dependent apoptosis. Proc Natl Acad Sci U S A 115(9):E2001–E2009. https://doi.org/10.1073/pnas.1722013115
Dondelinger Y, Delanghe T, Priem D, Wynosky-Dolfi MA, Sorobetea D, Rojas-Rivera D, Giansanti P, Roelandt R, Gropengiesser J, Ruckdeschel K, Savvides SN, Heck AJR, Vandenabeele P, Brodsky IE, Bertrand MJM (2019) Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation. Nat Commun 10(1):1729. https://doi.org/10.1038/s41467-019-09690-0
Wu XL, Hu H, Dong XQ, Zhang J, Wang J, Schwieters CD, Liu J, Wu GX, Li B, Lin JY, Wang HY, Lu JX (2021) The amyloid structure of mouse RIPK3 (receptor interacting protein kinase 3) in cell necroptosis. Nat Commun 12(1):1627. https://doi.org/10.1038/s41467-021-21881-2
Laurien L, Nagata M, Schünke H, Delanghe T, Wiederstein JL, Kumari S, Schwarzer R, Corona T, Krüger M, Bertrand MJM, Kondylis V, Pasparakis M (2020) Autophosphorylation at serine 166 regulates RIP kinase 1-mediated cell death and inflammation. Nat Commun 11(1):1747. https://doi.org/10.1038/s41467-020-15466-8
Chen X, Zhu R, Zhong J, Ying Y, Wang W, Cao Y, Cai H, Li X, Shuai J, Han J (2022) Mosaic composition of RIP1-RIP3 signalling hub and its role in regulating cell death. Nat Cell Biol 24(4):471–482. https://doi.org/10.1038/s41556-022-00854-7
Wang B, Fu J, Chai Y, Liu Y, Chen Y, Yin J, Pu Y, Chen C, Wang F, Liu Z, Zheng L, Chen M (2022) Accumulation of RIPK1 into mitochondria is requisite for oxidative stress-mediated necroptosis and proliferation in Rat Schwann cells. Int J Med Sci 19(13):1965–1976. https://doi.org/10.7150/ijms.69992
Hu S, Chang X, Zhu H, Wang D, Chen G (2020) PI3K mediates tumor necrosis factor induced-necroptosis through initiating RIP1-RIP3-MLKL signaling pathway activation. Cytokine 129:155046. https://doi.org/10.1016/j.cyto.2020.155046
Garnish SE, Meng Y, Koide A, Sandow JJ, Denbaum E, Jacobsen AV, Yeung W, Samson AL, Horne CR, Fitzgibbon C, Young SN, Smith PPC, Webb AI, Petrie EJ, Hildebrand JM, Kannan N, Czabotar PE, Koide S, Murphy JM (2021) Conformational interconversion of MLKL and disengagement from RIPK3 precede cell death by necroptosis. Nat Commun 12(1):2211. https://doi.org/10.1038/s41467-021-22400-z
Davies KA, Fitzgibbon C, Young SN, Garnish SE, Yeung W, Coursier D, Birkinshaw RW, Sandow JJ, Lehmann WIL, Liang LY, Lucet IS, Chalmers JD, Patrick WM, Kannan N, Petrie EJ, Czabotar PE, Murphy JM (2020) Distinct pseudokinase domain conformations underlie divergent activation mechanisms among vertebrate MLKL orthologues. Nat Commun 11(1):3060. https://doi.org/10.1038/s41467-020-16823-3
Bansal N, Sciabola S, Bhisetti G (2019) Understanding allosteric interactions in hMLKL protein that modulate necroptosis and its inhibition. Sci Rep 9(1):16853. https://doi.org/10.1038/s41598-019-53078-5
Petrie EJ, Sandow JJ, Jacobsen AV, Smith BJ, Griffin MDW, Lucet IS, Dai W, Young SN, Tanzer MC, Wardak A, Liang LY, Cowan AD, Hildebrand JM, Kersten WJA, Lessene G, Silke J, Czabotar PE, Webb AI, Murphy JM (2018) Conformational switching of the pseudokinase domain promotes human MLKL tetramerization and cell death by necroptosis. Nat Commun 9(1):2422. https://doi.org/10.1038/s41467-018-04714-7
Zhang Y, Liu J, Yu D, Zhu X, Liu X, Liao J, Li S, Wang H (2021) The MLKL kinase-like domain dimerization is an indispensable step of mammalian MLKL activation in necroptosis signaling. Cell Death Dis 12(7):638. https://doi.org/10.1038/s41419-021-03859-6
Davies KA, Tanzer MC, Griffin MDW, Mok YF, Young SN, Qin R, Petrie EJ, Czabotar PE, Silke J, Murphy JM (2018) The brace helices of MLKL mediate interdomain communication and oligomerisation to regulate cell death by necroptosis. Cell Death Differ 25(9):1567–1580. https://doi.org/10.1038/s41418-018-0061-3
Zhu X, Yang N, Yang Y, Yuan F, Yu D, Zhang Y, Shu Z, Nan N, Hu H, Liu X, Chen S, Sun L, Wang H (2022) Spontaneous necroptosis and autoinflammation are blocked by an inhibitory phosphorylation on MLKL during neonatal development. Cell Res 32(4):407–410. https://doi.org/10.1038/s41422-021-00583-w
Dai J, Zhang C, Guo L, He H, Jiang K, Huang Y, Zhang X, Zhang H, Wei W, Zhang Y, Lu L, Hu J (2020) A necroptotic-independent function of MLKL in regulating endothelial cell adhesion molecule expression. Cell Death Dis 11(4):282. https://doi.org/10.1038/s41419-020-2483-3
Samson AL, Zhang Y, Geoghegan ND, Gavin XJ, Davies KA, Mlodzianoski MJ, Whitehead LW, Frank D, Garnish SE, Fitzgibbon C, Hempel A, Young SN, Jacobsen AV, Cawthorne W, Petrie EJ, Faux MC, Shield-Artin K, Lalaoui N, Hildebrand JM, Silke J, Rogers KL, Lessene G, Hawkins ED, Murphy JM (2020) MLKL trafficking and accumulation at the plasma membrane control the kinetics and threshold for necroptosis. Nat Commun 11(1):3151. https://doi.org/10.1038/s41467-020-16887-1
Ruiz K, Thaker TM, Agnew C, Miller-Vedam L, Trenker R, Herrera C, Ingaramo M, Toso D, Frost A, Jura N (2019) Functional role of PGAM5 multimeric assemblies and their polymerization into filaments. Nat Commun 10(1):531. https://doi.org/10.1038/s41467-019-08393-w
Chen Y, Gong K, Guo L, Zhang B, Chen S, Li Z, Quanhua X, Liu W, Wang Z (2021) Downregulation of phosphoglycerate mutase 5 improves microglial inflammasome activation after traumatic brain injury. Cell Death Discov 7(1):290. https://doi.org/10.1038/s41420-021-00686-8
Dai C, Qu B, Peng B, Liu B, Li Y, Niu C, Peng B, Li D (2023) Phosphoglycerate mutase 5 facilitates mitochondrial dysfunction and neuroinflammation in spinal tissues after spinal cord injury. Int Immunopharmacol 116:109773. https://doi.org/10.1016/j.intimp.2023.109773
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y (2018) Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 163–164:144–171. https://doi.org/10.1016/j.pneurobio.2017.10.001
Yang J, Zhao Y, Zhang L, Fan H, Qi C, Zhang K, Liu X, Fei L, Chen S, Wang M, Kuang F, Wang Y, Wu S (2018) RIPK3/MLKL-Mediated neuronal necroptosis modulates the M1/M2 polarization of Microglia/Macrophages in the ischemic cortex. Cereb Cortex 28(7):2622–2635. https://doi.org/10.1093/cercor/bhy089
Chen AQ, Fang Z, Chen XL, Yang S, Zhou YF, Mao L, Xia YP, Jin HJ, Li YN, You MF, Wang XX, Lei H, He QW, Hu B (2019) Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis 10(7):487. https://doi.org/10.1038/s41419-019-1716-9
Fan H, Zhang K, Shan L, Kuang F, Chen K, Zhu K, Ma H, Ju G, Wang YZ (2016) Reactive astrocytes undergo M1 microglia/macrohpages-induced necroptosis in spinal cord injury. Mol Neurodegener 11:14. https://doi.org/10.1186/s13024-016-0081-8
Ni Y, Gu WW, Liu ZH, Zhu YM, Rong JG, Kent TA, Li M, Qiao SG, An JZ, Zhang HL (2018) RIP1K contributes to neuronal and astrocytic cell death in ischemic stroke via activating autophagic-lysosomal pathway. Neuroscience 371:60–74. https://doi.org/10.1016/j.neuroscience.2017.10.038
Xu Y, Wu X, Hu W, Yu D, Shao Z, Li W, Huang T, Zhang J, Zhu X, Li X, Yang H, Chu Z, Lv K (2021) RIP3 facilitates necroptosis through CaMKII and AIF after intracerebral hemorrhage in mice. Neurosci Lett 749:135699. https://doi.org/10.1016/j.neulet.2021.135699
Lin QS, Chen P, Wang WX, Lin CC, Zhou Y, Yu LH, Lin YX, Xu YF, Kang DZ (2020) RIP1/RIP3/MLKL mediates dopaminergic neuron necroptosis in a mouse model of Parkinson disease. Lab Invest 100(3):503–511. https://doi.org/10.1038/s41374-019-0319-5
Wang X, Mao X, Liang K, Chen X, Yue B, Yang Y (2021) RIP3-mediated necroptosis was essential for spiral ganglion neuron damage. Neurosci Lett 744:135565. https://doi.org/10.1016/j.neulet.2020.135565
Wang Y, Jiao J, Zhang S, Zheng C, Wu M (2019) RIP3 inhibition protects locomotion function through ameliorating mitochondrial antioxidative capacity after spinal cord injury. Biomed Pharmacother 116:109019. https://doi.org/10.1016/j.biopha.2019.109019
Zhao X, Lu J, Chen X, Gao Z, Zhang C, Chen C, Qiao D, Wang H (2021) Methamphetamine exposure induces neuronal programmed necrosis by activating the receptor-interacting protein kinase 3 -related signalling pathway. FASEB J 35(5):e21561. https://doi.org/10.1096/fj.202100188R
Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ (2011) Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol 70(3):374–383. https://doi.org/10.1002/ana.22455
Huang Z, Liang J, Chen S, Ng TK, Brelén ME, Liu Q, Yang R, Xie B, Ke S, Chen W, Huang D (2023) RIP3-mediated microglial necroptosis promotes neuroinflammation and neurodegeneration in the early stages of diabetic retinopathy. Cell Death Dis 14(3):227. https://doi.org/10.1038/s41419-023-05660-z
Zhang L, Feng Q, Wang T (2018) Necrostatin-1 protects against Paraquat-Induced Cardiac Contractile Dysfunction via RIP1-RIP3-MLKL-Dependent necroptosis pathway. Cardiovasc Toxicol 18(4):346–355. https://doi.org/10.1007/s12012-017-9441-z
Wei J, Chen L, Wang D, Tang L, Xie Z, Chen W, Zhang S, Weng G (2021) Upregulation of RIP3 promotes necroptosis via a ROS–dependent NF–κB pathway to induce chronic inflammation in HK–2 cells. Mol Med Rep 24(5):783. https://doi.org/10.3892/mmr.2021.12423
Cao M, Chen F, Xie N, Cao MY, Chen P, Lou Q, Zhao Y, He C, Zhang S, Song X, Sun Y, Zhu W, Mou L, Luan S, Gao H (2018) c-Jun N-terminal kinases differentially regulate TNF- and TLRs-mediated necroptosis through their kinase-dependent and -independent activities. Cell Death Dis 9(12):1140. https://doi.org/10.1038/s41419-018-1189-2
Huang S, Hu W, Rao D, Wu X, Bai Q, Wang J, Chu Z, Xu Y (2022) RIPK3-Dependent necroptosis activates MCP-1-Mediated inflammation in mice after Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis 31(1):106213. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106213
Liu C, Chen Y, Cui W, Cao Y, Zhao L, Wang H, Liu X, Fan S, Huang K, Tong A, Zhou L (2021) Inhibition of neuronal necroptosis mediated by RIP1/RIP3/MLKL provides neuroprotective effects on kaolin-induced hydrocephalus in mice. Cell Prolif 54(9):e13108. https://doi.org/10.1111/cpr.13108
Wu W, Wang X, Sun Y, Berleth N, Deitersen J, Schlütermann D, Stuhldreier F, Wallot-Hieke N, José Mendiburo M, Cox J, Peter C, Bergmann AK, Stork B (2021) TNF-induced necroptosis initiates early autophagy events via RIPK3-dependent AMPK activation, but inhibits late autophagy. Autophagy 17(12):3992–4009. https://doi.org/10.1080/15548627.2021.1899667
Cruz SA, Qin Z, Stewart AFR, Chen HH (2018) Dabrafenib, an inhibitor of RIP3 kinase-dependent necroptosis, reduces ischemic brain injury. Neural Regen Res 13(2):252–256. https://doi.org/10.4103/1673-5374.226394
https://www.cancer.gov/news-events/cancer-currents-blog/2022/fda-dabrafenib-trametinib-braf-solid-tumors
Xia K, Zhu F, Yang C, Wu S, Lin Y, Ma H, Yu X, Zhao C, Ji Y, Ge W, Wang J, Du Y, Zhang W, Yang T, Zhang X, He S (2020) Discovery of a potent RIPK3 inhibitor for the amelioration of Necroptosis-Associated Inflammatory Injury. Front Cell Dev Biol 8:606119. https://doi.org/10.3389/fcell.2020.606119
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (no. LY19H170001).
Author information
Authors and Affiliations
Contributions
Xuehong Liu designed the study. Lvxia Wang, Yong Zhang, Min Huang and Xuehong Liu prepared the first draft of the manuscript. Lvxia Wang, Yong Zhang, Min Huang and Xuehong Liu revised the manuscript. All authors approved the final paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed to publish this article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Zhang, Y., Huang, M. et al. RIP3 in Necroptosis: Underlying Contributions to Traumatic Brain Injury. Neurochem Res 49, 245–257 (2024). https://doi.org/10.1007/s11064-023-04038-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04038-z