Abstract
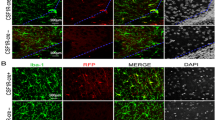
Periventricular leukomalacia (PVL), a predominant form of brain injury in preterm survivors, is characterized by hypomyelination and microgliosis and is also the major cause of long-term neurobehavioral abnormalities in premature infants. Receptor-interacting protein kinase 1 (RIPK1) plays a pivotal role in mediating cell death and inflammatory signaling cascade. However, very little is known about the potential effect of RIPK1 in PVL and the underlying mechanism. Herein, we found that the expression level of RIPK1 was drastically increased in the brain of PVL neonatal mice models, and treatment of PVL neonatal mice with Necrostatin-1s (Nec-1s), an inhibitor of RIPK1, greatly ameliorated cerebral ischemic injury, exhibiting an increase of body weights, reduction of cerebral infarct size, neuronal loss, and occurrence of necrosis-like cells, and significantly improved the long-term abnormal neurobehaviors of PVL. In addition, Nec-1s significantly suppressed hypomyelination and promoted the differentiation of oligodendrocyte precursor cells (OPCs), as demonstrated by the increased expression levels of MBP and Olig2, the decreased expression level of GPR17, a significant increase in the number of CC-1-positive cells, and suppression of myelin ultrastructure impairment. Moreover, the mechanism of neuroprotective effects of Nec-1s against PVL is closely associated with its suppression of the RIPK1-mediated necrosis signaling molecules, RIPK1, PIPK3, and MLKL. More importantly, inhibition of RIPK1 could reduce microglial inflammatory injury by triggering the M1 to M2 microglial phenotype, appreciably decreasing the levels of M1 marker CD86 and increasing the levels of M2 markers Arg1 or CD206 in PVL mice. Taken together, inhibition of RIPK1 markedly ameliorates the brain injury and long-term neurobehavioral abnormalities of PVL mice through the reduction of neural cell necroptosis and reversing neuroinflammation.








Similar content being viewed by others
Change history
13 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11064-023-04027-2
References
Litt J, Taylor HG, Klein N, Hack M (2005) Learning disabilities in children with very low birthweight: prevalence, neuropsychological correlates, and educational interventions. J Learn Disabil 38:130–141. https://doi.org/10.1177/00222194050380020301
Soria-Pastor S, Gimenez M, Narberhaus A, Falcon C, Botet F, Bargallo N, Mercader JM, Junque C (2008) Patterns of cerebral white matter damage and cognitive impairment in adolescents born very preterm. Int J Dev Neurosci 26:647–654. https://doi.org/10.1016/j.ijdevneu.2008.08.001
Vanes LD, Murray RM, Nosarti C (2022) Adult outcome of preterm birth: implications for neurodevelopmental theories of psychosis. Schizophr Res 247:41–54. https://doi.org/10.1016/j.schres.2021.04.007
Back SA (2017) White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol 134:331–349. https://doi.org/10.1007/s00401-017-1718-6
Galinsky R, Lear CA, Dean JM, Wassink G, Dhillon SK, Fraser M, Davidson JO, Bennet L, Gunn AJ (2018) Complex interactions between hypoxia-ischemia and inflammation in preterm brain injury. Dev Med Child Neurol 60:126–133. https://doi.org/10.1111/dmcn.13629
Zaghloul N, Ahmed M (2017) Pathophysiology of periventricular leukomalacia: what we learned from animal models. Neural Regen Res 12:1795–1796. https://doi.org/10.4103/1673-5374.219034
Yuan J, Amin P, Ofengeim D (2019) Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci 20:19–33. https://doi.org/10.1038/s41583-018-0093-1
Zelic M, Pontarelli F, Woodworth L, Zhu C, Mahan A, Ren Y, Lamorte M, Gruber R, Keane A, Loring P, Guo L, Xia TH, Zhang B, Orning P, Lien E, Degterev A, Hammond T, Ofengeim D (2021) RIPK1 activation mediates neuroinflammation and disease progression in multiple sclerosis. Cell Rep 35:109112. https://doi.org/10.1016/j.celrep.2021.109112
Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, Hitomi J, Zhu H, Chen H, Mayo L, Geng J, Amin P, Dewitt JP, Mookhtiar AK, Florez M, Ouchida AT, Fan JB, Pasparakis M, Kelliher MA, Ravits J, Yuan J (2016) RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 353:603–608. https://doi.org/10.1126/science.aaf6803
Deng X, Li S, Sun F (2019) Necrostatin-1 prevents necroptosis in brains after ischemic stroke via inhibition of RIPK1-Mediated RIPK3/MLKL signaling. Aging Dis 10:807. https://doi.org/10.14336/AD.2018.0728
Liu J, Hu H, Wu B (2021) RIPK1 inhibitor ameliorates the MPP(+)/MPTP-induced Parkinson’s disease through the ASK1/JNK signalling pathway. Brain Res 1757:147310. https://doi.org/10.1016/j.brainres.2021.147310
Li S, Qu L, Wang X, Kong L (2022) Novel insights into RIPK1 as a promising target for future Alzheimer’s disease treatment. Pharmacol Ther 231:107979. https://doi.org/10.1016/j.pharmthera.2021.107979
Back SA, Luo NL, Borenstein NS, Volpe JJ, Kinney HC (2002) Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol 61:197–211. https://doi.org/10.1093/jnen/61.2.197
Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM (2002) Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci 22:455–463. https://doi.org/10.1523/JNEUROSCI.22-02-00455.2002
Qu Y, Tang J, Wang H, Li S, Zhao F, Zhang L, Richard LQ, Mu D (2017) RIPK3 interactions with MLKL and CaMKII mediate oligodendrocytes death in the developing brain. Cell Death Dis 8:e2629. https://doi.org/10.1038/cddis.2017.54
Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ (2011) Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab 31:178–189. https://doi.org/10.1038/jcbfm.2010.72
Wang J, Wang D, Zheng X, Li Y, Li Y, Ma T, Li J, Sun J, Wang Y, Ma Q (2022) A2B adenosine receptor inhibition ameliorates hypoxic-ischemic injury in neonatal mice via PKC/Erk/Creb/HIF-1alpha signaling pathway. Brain Res 1782:147837. https://doi.org/10.1016/j.brainres.2022.147837
Reddy N, Doyle M, Hanagandi P, Taranath A, Dahmoush H, Krishnan P, Oztekin O, Boltshauser E, Shroff M, Mankad K (2022) Neuroradiological mimics of Periventricular Leukomalacia. J Child Neurol 37:151–167. https://doi.org/10.1177/08830738211026052
Khurana R, Shyamsundar K, Taank P, Singh A (2021) Periventricular leukomalacia: an ophthalmic perspective. Med J Armed Forces India 77:147–153. https://doi.org/10.1016/j.mjafi.2020.05.013
Martinez-Biarge M, Groenendaal F, Kersbergen KJ, Benders M, Foti F, van Haastert IC, Cowan FM, de Vries LS (2019) Neurodevelopmental outcomes in Preterm Infants with White Matter Injury using a new MRI classification. Neonatology 116:227–235. https://doi.org/10.1159/000499346
Lecca D, Raffaele S, Abbracchio MP, Fumagalli M (2020) Regulation and signaling of the GPR17 receptor in oligodendroglial cells. Glia 68:1957–1967. https://doi.org/10.1002/glia.23807
Wang J, He X, Meng H, Li Y, Dmitriev P, Tian F, Page JC, Lu QR, He Z (2020) Robust myelination of regenerated axons Induced by Combined Manipulations of GPR17 and Microglia. Neuron 108:876–886. https://doi.org/10.1016/j.neuron.2020.09.016
Merten N, Fischer J, Simon K, Zhang L, Schroder R, Peters L, Letombe AG, Hennen S, Schrage R, Bodefeld T, Vermeiren C, Gillard M, Mohr K, Lu QR, Brustle O, Gomeza J, Kostenis E (2018) Repurposing HAMI3379 to Block GPR17 and promote rodent and human oligodendrocyte differentiation. Cell Chem Biol 25:775–786. https://doi.org/10.1016/j.chembiol.2018.03.012
Wang J, Yang L, Jiang M, Zhao C, Liu X, Berry K, Waisman A, Langseth AJ, Novitch BG, Bergles DE, Nishiyama A, Lu QR (2022) Olig2 ablation in immature oligodendrocytes does not enhance CNS myelination and remyelination. J Neurosci 42:8542–8555. https://doi.org/10.1523/JNEUROSCI.0237-22.2022
Hammond TR, Robinton D, Stevens B (2018) Microglia and the brain: complementary partners in Development and Disease. Annu Rev Cell Dev Biol 34:523–544. https://doi.org/10.1146/annurev-cellbio-100616-060509
Shao R, Sun D, Hu Y, Cui D (2021) White matter injury in the neonatal hypoxic-ischemic brain and potential therapies targeting microglia. J Neurosci Res 99:991–1008. https://doi.org/10.1002/jnr.24761
Volpe JJ (2019) Microglia: newly discovered complexity could lead to targeted therapy for neonatal white matter injury and dysmaturation. J Neonatal Perinatal Med 12:239–242. https://doi.org/10.3233/NPM-190303
Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z (2005) Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience 133:159–168. https://doi.org/10.1016/j.neuroscience.2005.02.016
Pang Y, Fan LW, Tien LT, Dai X, Zheng B, Cai Z, Lin RC, Bhatt A (2013) Differential roles of astrocyte and microglia in supporting oligodendrocyte development and myelination in vitro. Brain Behav 3:503–514. https://doi.org/10.1002/brb3.152
Pang Y, Cai Z, Rhodes PG (2003) Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res 140:205–214. https://doi.org/10.1016/s0165-3806(02)00606-5
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin R, Ffrench-Constant C (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16:1211–1218. https://doi.org/10.1038/nn.3469
Sherwin C, Fern R (2005) Acute lipopolysaccharide-mediated injury in neonatal white matter glia: role of TNF-alpha, IL-1beta, and calcium. J Immunol 175:155–161. https://doi.org/10.4049/jimmunol.175.1.155
Anderson PJ, De Luca CR, Hutchinson E, Spencer-Smith MM, Roberts G, Doyle LW (2011) Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev Neuropsychol 36:57–73. https://doi.org/10.1080/87565641.2011.540538
Liu J, Li J, Qin GL, Chen YH, Wang Q (2008) Periventricular leukomalacia in premature infants in mainland China. Am J Perinatol 25:535–540. https://doi.org/10.1055/s-0028-1083841
Jacobson LK, Dutton GN (2000) Periventricular leukomalacia: an important cause of visual and ocular motility dysfunction in children. Surv Ophthalmol 45:1–13. https://doi.org/10.1016/s0039-6257(00)00134-x
Chen Y, Zhang L, Yu H, Song K, Shi J, Chen L, Cheng J (2018) Necrostatin-1 improves long-term functional recovery through protecting oligodendrocyte precursor cells after transient focal cerebral ischemia in mice. Neuroscience 371:229–241. https://doi.org/10.1016/j.neuroscience.2017.12.007
Gao X, Zhang P, Chen J, Zhang L, Shang N, Chen J, Fan R, Wang Y, Huang T, Niu Q, Zhang Q (2022) Necrostatin-1 relieves learning and memory deficits in a zebrafish model of Alzheimer’s Disease Induced by Aluminum. Neurotox Res 40:198–214. https://doi.org/10.1007/s12640-021-00463-6
Funding
This work was funded by the National Natural Science Foundation of China (grant nos.82060223, 32160192), the Natural Science Foundation of Ningxia (grant nos.2022AAC03607, 2023AAC02037, 2023AAC03162), the Key Research and Development Plan of Ningxia (grant no. 2021BEG03097).
Author information
Authors and Affiliations
Contributions
Jinping Sun contributed to the acquisition, analysis, and interpretation of data, the original manuscript. Jinping Sun, Xiaoli Pan, and Hualiang Zhai performed the experiments. Junyan Wang participated in the conception of the study and supervised the graduate students. Yong Han participated in the data analysis and chart making. Quanrui Ma contributed to the project design and the manuscript revision. Yunhong Li, Wei Wang, and Yin Wang contributed to the funding support, project design, and supervision, the manuscript revision. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jinping Sun and Wei Wang contributed equally.
The original online version of this article was revised: due to missing equal contributor information.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, J., Wang, W., Ma, Q. et al. Necrostatin-1s Suppresses RIPK1-driven Necroptosis and Inflammation in Periventricular Leukomalacia Neonatal Mice. Neurochem Res 49, 129–141 (2024). https://doi.org/10.1007/s11064-023-04013-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04013-8