Abstract
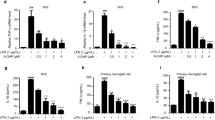
Neuroinflammation is a critical driver in the pathogenesis and progression of neurodegenerative disorders. Dammarane sapogenins (DS), a deglycosylated product of ginsenoside, possess a variety of potent biological activities. The present study aimed to explore the neuroprotective effects of DS in a rat model of neuroinflammation induced by intracerebroventricular injection of lipopolysaccharide (LPS). Our study revealed that DS pretreatment effectively improved LPS-induced associative learning and memory impairments in the active avoidance response test and spatial learning and memory in Morris water maze test. DS also remarkably inhibited LPS-induced neuroinflammation by suppressing microglia overactivation, pro-inflammatory cytok ine release (TNF‐α and IL‐1β) and reducing neuronal loss in the CA1 and DG regions of the hippocampus. Importantly, pretreatment with DS reversed LPS-induced upregulation of HMGB1 and TLR4 and inhibited their downstream NF-κB signaling activation, as evidenced by increased IκBα and decreased p-NF‐κB p65 levels. Furthermore, DS ameliorated LPS-induced synaptic dysfunction by decreasing MMP-9 and increasing NMDAR1 expression in the hippocampus. Taken together, this study suggests that DS could be a promising treatment for preventing cognitive impairments caused by neuroinflammation.









Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Azam S, Haque ME, Jakaria M, Jo SH, Kim IS, Choi DK (2020) G-protein-coupled receptors in CNS: a potential therapeutic target for intervention in neurodegenerative disorders and associated cognitive deficits. Cells 9:506. https://doi.org/10.1186/s12974-014-0151-1
Cotter J, Granger K, Backx R, Hobbs M, Looi CY, Barnett JH (2018) Social cognitive dysfunction as a clinical marker: a systematic review of meta-analyses across 30 clinical conditions. Neurosci Biobehav Rev 84:92–99. https://doi.org/10.5114/fn.2019.84423
2021 Alzheimer’s disease facts and Fig. (2021) Alzheimers Dement 17:327–406. https://doi.org/10.1002/alz.12328
Allison DJ, Ditor DS (2014) The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J Neuroinflammation 11:151. https://doi.org/10.1186/s12974-014-0151-1
Wang J, Song Y, Chen Z, Leng SX (2018) Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid Med Cell Longev 2018, 1972714. https://doi.org/10.1155/2018/1972714
Bradburn S, Murgatroyd C, Ray N (2019) Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. Ageing Res Rev 50:1–8. https://doi.org/10.1016/j.arr.2019.01.002
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12:719–732. https://doi.org/10.1016/j.jalz.2016.02.010
Feng X, Hu J, Zhan F, Luo D, Hua F, Xu G (2021) MicroRNA-138-5p regulates hippocampal neuroinflammation and cognitive impairment by NLRP3/Caspase-1 signaling pathway in rats. J Inflamm Res 14:1125–1143. https://doi.org/10.2147/JIR.S304461
Mao X, Kelty TJ, Kerr NR, Childs TE, Roberts MD, Booth FW (2021) Creatine supplementation upregulates mTORC1 signaling and markers of synaptic plasticity in the dentate gyrus while ameliorating LPS-induced cognitive impairment in female rats. Nutrients 13:2758. https://doi.org/10.3390/nu13082758
Hainmueller T, Bartos M (2018) Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558:292–296. https://doi.org/10.1038/s41586-018-0191-2
Cornell J, Salinas S, Huang HY, Zhou M (2022) Microglia regulation of synaptic plasticity and learning and memory. Neural Regen Res 17:705–716. https://doi.org/10.4103/1673-5374.322423
Cunningham C (2013) Microglia and neurodegeneration: the role of systemic inflammation. Glia 61:71–90. https://doi.org/10.1002/glia.22350
Szczepanik AM, Ringheim GE (2003) IL-10 and glucocorticoids inhibit Abeta (1–42) and lipopolysaccharide-induced pro-inflammatory cytokine and chemokine induction in the central nervous system. J Alzheimers Dis 5:105–117. https://doi.org/10.3233/jad-2003-5205
Paudel YN, Shaikh MF, Chakraborti A, Kumari Y, Aledo-Serrano Á, Aleksovska K, Alvim MKM, Othman I (2018) HMGB1: a common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front Neurosci 12:628. https://doi.org/10.3389/fnins.2018.00628
Fujita K, Motoki K, Tagawa K, Chen X, Hama H, Nakajima K, Homma H, Tamura T, Watanabe H, Katsuno M, Matsumi C, Kajikawa M, Saito T, Saido T, Sobue G, Miyawaki A, Okazawa H (2016) HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease. Sci Rep 6:31895. https://doi.org/10.1038/srep31895
Sasaki T, Liu K, Agari T, Yasuhara T, Morimoto J, Okazaki M, Takeuchi H, Toyoshima A, Sasada S, Shinko A, Kondo A, Kameda M, Miyazaki I, Asanuma M, Borlongan CV, Nishibori M, Date I (2016) Anti-high mobility group box 1 antibody exerts neuroprotection in a rat model of Parkinson’s disease. Exp Neurol 275 Pt 1220–231. https://doi.org/10.1016/j.expneurol.2015.11.003
Ravizza T, Terrone G, Salamone A, Frigerio F, Balosso S, Antoine DJ, Vezzani A (2017) High mobility group box 1 is a novel pathogenic factor and a mechanistic biomarker for epilepsy. Brain Behav Immun 72:14–21. https://doi.org/10.1016/j.bbi.2017.10.008
Meng L, Li L, Lu S, Li K, Su Z, Wang Y, Fan X, Li X, Zhao G (2018) The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways. Mol Immunol 94:7–17. https://doi.org/10.1016/j.molimm.2017.12.008
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39. https://doi.org/10.1038/361031a0
Ju Y, Tam KY (2022) Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen Res 17:543–549. https://doi.org/10.4103/1673-5374.320970
Vafadari B, Salamian A, Kaczmarek L (2016) MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J Neurochem 139(2):91–114. https://doi.org/10.1111/jnc.13415
Elmore MRP, Hohsfield LA, Kramár EA, Soreq L, Lee RJ, Pham ST, Najafi AR, Spangenberg EE, Wood MA, West BL, Green KN (2018) Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 17:e12832. https://doi.org/10.1111/acel.12832
Kim HJ, Jung SW, Kim SY, Cho IH, Kim HC, Rhim H, Kim M, Nah SY (2018) Panax ginseng as an adjuvant treatment for Alzheimer’s disease. J Ginseng Res 42:401–411. https://doi.org/10.1016/j.jgr.2017.12.008
Huang X, Li N, Pu Y, Zhang T, Wang B (2019) Neuroprotective effects of ginseng phytochemicals: recent perspectives. Molecules 24:2939. https://doi.org/10.3390/molecules24162939
Feng H, Xue M, Deng H, Cheng S, Hu Y, Zhou C (2022) Ginsenoside and its therapeutic potential for cognitive impairment. Biomolecules 12:1310. https://doi.org/10.3390/biom12091310
Mohanan P, Subramaniyam S, Mathiyalagan R, Yang DC (2018) Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res 42:123–132. https://doi.org/10.1016/j.jgr.2017.01.008
Piao X, Zhang H, Kang JP, Yang DU, Li Y, Pang S, Jin Y, Yang DC, Wang Y (2020) Advances in saponin diversity of Panax ginseng. Molecules 25:3452. https://doi.org/10.3390/molecules25153452
Dong L, Yang Y, Lu Y, Lu C, Lv J, Jiang N, Xu Q, Gao Y, Chang Q, Liu X (2018) Radioprotective effects of dammarane sapogenins against 60 co-induced myelosuppression in mice. Phytother Res 32:741–749. https://doi.org/10.1002/ptr.6027
Jiang N, Zhang BY, Dong LM, Lv JW, Lu C, Wang Q, Fan LX, Zhang HX, Pan RL, Liu XM (2018) Antidepressant effects of dammarane sapogenins in chronic unpredictable mild stress-induced depressive mice. Phytother Res 32:1023–1029. https://doi.org/10.1002/ptr.6040
Wang Q, Dong L, Wang M, Chen S, Li S, Chen Y, He W, Zhang H, Zhang Y, Pires Dias AC, Yang S, Liu X (2021) Dammarane sapogenins improving simulated weightlessness-induced depressive-like behaviors and cognitive dysfunction in rats. Front Psychiatry 12:638328. https://doi.org/10.3389/fpsyt.2021.638328
Dong L, Wang Y, Lv J, Zhang H, Jiang N, Lu C, Xu P, Liu X (2019) Memory enhancement of fresh ginseng on deficits induced by chronic restraint stress in mice. Nutr Neurosci 22:235–242. https://doi.org/10.1080/1028415X.2017.1373928
Liu W, Liu J, Gao J, Duan X, Zhang L (2022) Effects of subchronic aluminum exposure on learning, memory, and neurotrophic factors in rats. Neurotox Res 40:2046–2060. https://doi.org/10.1007/s12640-022-00599-z
Lee YJ, Choi DY, Choi IS, Kim KH, Kim YH, Kim HM, Lee K, Cho WG, Jung JK, Han SB, Han JY, Nam SY, Yun YW, Jeong JH, Oh KW, Hong JT (2012) Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappab in vitro and in vivo models. J Neuroinflammation 9:35. https://doi.org/10.1186/1742-2094-9-35
García-Capdevila S, Portell-Cortés I, Torras-Garcia M, Coll-Andreu M, Costa-Miserachs D (2009) Effects of long-term voluntary exercise on learning and memory processes: dependency of the task and level of exercise. Behav Brain Res 202:162–170. https://doi.org/10.1016/j.bbr.2009.03.020
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36:60–90. https://doi.org/10.1016/s0165-0173(01)00067-4
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Attia GM, Elmansy RA, Elsaed WM (2019) Neuroprotective effect of nilotinib on pentylenetetrazol-induced epilepsy in adult rat hippocampus: involvement of oxidative stress, autophagy, inflammation, and apoptosis. Folia Neuropathol 57:146–160. https://doi.org/10.5114/fn.2019.84423
Reagan LP, McEwen BS (1997) Controversies surrounding glucocorticoid-mediated cell death in the hippocampus. J Chem Neuroanat 13:149–167. https://doi.org/10.1016/s0891-0618(97)00031-8
Owen JE, BenediktsdÓttir B, Gislason T, Robinson SR (2019) Neuropathological investigation of cell layer thickness and myelination in the hippocampus of people with obstructive sleep apnea. Sleep 42. https://doi.org/10.1093/sleep/zsy199
Mirescu C, Gould E (2006) Stress and adult neurogenesis. Hippocampus 16:233–238. https://doi.org/10.1002/hipo.20155
Kreisman NR, Soliman S, Gozal D (2000) Regional differences in hypoxic depolarization and swelling in hippocampal slices. J Neurophysiol 83:1031–1038. https://doi.org/10.1152/jn.2000.83.2.1031
Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T (2003) Activation of innate immunity in the CNS triggers neurodegeneration through a toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A 100:8514–8519. https://doi.org/10.1152/jn.2000.83.2.1031
Li Y, Liu T, Li Y, Han D, Hong J, Yang N, He J, Peng R, Mi X, Kuang C, Zhou Y, Han Y, Shi C, Li Z, Guo X (2020) Baicalin ameliorates cognitive impairment and protects microglia from LPS-induced neuroinflammation via the SIRT1/HMGB1 pathway. Oxid Med Cell Longev 2020, 4751349. https://doi.org/10.1155/2020/4751349
Wang L, Yang JW, Lin LT, Huang J, Wang XR, Su XT, Cao Y, Fisher M, Liu CZ (2020) Acupuncture attenuates inflammation in microglia of vascular dementia rats by inhibiting miR-93-mediated TLR4/MyD88/NF-κB signaling pathway. Oxid Med Cell Longev 2020, 8253904. https://doi.org/10.1155/2020/8253904
Fang P, Schachner M, Shen YQ (2012) HMGB1 in development and diseases of the central nervous system. Mol Neurobiol 45:499–506. https://doi.org/10.1007/s12035-012-8264-y
Hayden MS, Ghosh S (2011) NF-κB in immunobiology. Cell Res 21:223–244. https://doi.org/10.1038/cr.2011.13
Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR (1998) Matrix metalloproteinases and diseases of the CNS. Trends Neurosci 21:75–80. https://doi.org/10.1016/s0166-2236(97)01169-7
He C, Zhao X, Li H, Wang F, Zhang J, Wang Y, Han Y, Yuan C, Niu Q (2021) Regulation of mGluR1 on the expression of PKC and NMDAR in aluminum-exposed PC12 cells. Neurotox Res 39:634–644. https://doi.org/10.1007/s12640-020-00319-5
Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44:5–21. https://doi.org/10.1016/j.neuron.2004.09.012
Lau CG, Zukin RS (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8:413–426. https://doi.org/10.1038/nrn2153
Funding
This work was supported by Beijing Tongren Hospital, Capital Medical University (2020-YJJ-ZZL-050), Bethune Public Welfare Foundation (B-19-H-20200622) and Chinese Academy of Medical Sciences and Peking Union Medical College (2016-I2M-2-006).
Author information
Authors and Affiliations
Contributions
Liming Dong performed the experiments and prepared the manuscript. Ning Jiang and Jie Bai provided experimental technical support. Yiman Li and Zhihui Song performed the data analysis. Xinmin Liu and Chao Zhang conceived the experiments and revised the manuscript. All authors checked and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests.
This study was performed in line with the guidelines for the Animal Care and Use Committee of the Institute of Medical Plant Development, Chinese Academy of Medical Sciences, and Peking Union Medical College.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, L., Jiang, N., Bai, J. et al. Neuroprotective Effects of Dammarane Sapogenins Against lipopolysaccharide-induced Cognitive Impairment, Neuroinflammation and Synaptic Dysfunction. Neurochem Res 48, 3525–3537 (2023). https://doi.org/10.1007/s11064-023-03997-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03997-7