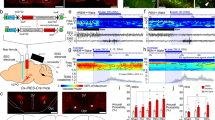
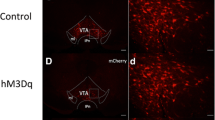
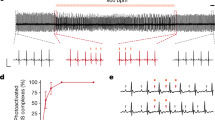
Abstract
Insomnia and anxiety are two common and closely related clinical problems that pose a threat to individuals’ physical and mental well-being. There is a possibility that some nuclei and neural circuits in the brain are shared by both insomnia and anxiety. In the present study, using a combination of chemogenetics, optogenetics, polysomnographic recordings and the classic tests of anxiety-like behaviors, we verified that the calmodulin-dependent protein kinase II alpha (CaMKIIa) neurons of the ventromedial hypothalamus (VMH) are involved in the regulation of both wakefulness and anxiety. Chemogenetic manipulation of the VMH CaMKIIa neurons elicited an apparent increase in wakefulness during activation, whereas inhibition decreased wakefulness mildly. It substantiated that the VMH CaMKIIa neurons contribute to wakefulness. Then in millisecond-scale control of neuronal activity, short-term and long-term optogenetic activation induced the initiation and maintenance of wakefulness, respectively. We also observed that mice reduced exploratory behaviors in classic anxiety tests while activating the VMH CaMKIIa neurons and were anxiolytic while inhibiting. Additionally, photostimulation of the VMH CaMKIIa axons in the paraventricular hypothalamus (PVH) mediated wakefulness and triggered anxiety-like behaviors as well. In conclusion, our results demonstrate that the VMH participates in the control of wakefulness and anxiety, and offer a neurological explanation for insomnia and anxiety, which may be valuable for therapeutic interventions such as medication and transcranial magnetic stimulation.




Similar content being viewed by others
Data Availability
Data supporting the results of this study are available from the corresponding author upon reasonable request.
References
Johnson EO, Roth T, Breslau N (2006) The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res 40(8):700–708. https://doi.org/10.1016/j.jpsychires.2006.07.008
Blake MJ, Trinder JA, Allen NB (2018) Mechanisms underlying the association between insomnia, anxiety, and depression in adolescence: implications for behavioral sleep interventions. Clin Psychol Rev 63:25–40. https://doi.org/10.1016/j.cpr.2018.05.006
Chellappa SL, Aeschbach D (2022) Sleep and anxiety: from mechanisms to interventions. Sleep Med Rev 61:101583. https://doi.org/10.1016/j.smrv.2021.101583
Vadnie CA, Petersen KA, Eberhardt LA, Hildebrand MA, Cerwensky AJ, Zhang H, Burns JN, Becker-Krail DD, DePoy LM, Logan RW, McClung CA (2021) The Suprachiasmatic Nucleus regulates anxiety-like Behavior in mice. Front NeuroSci 15:765850. https://doi.org/10.3389/fnins.2021.765850
Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, Jansson-Fröjmark M, Palagini L, Rücker G, Riemann D, Baglioni C (2019) Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev 43:96–105. https://doi.org/10.1016/j.smrv.2018.10.006
Kobayashi I, Boarts JM, Delahanty DL (2007) Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology 44(4):660–669. https://doi.org/10.1111/j.1469-8986.2007.537.x
Tseng YT, Zhao B, Chen S, Ye J, Liu J, Liang L, Ding H, Schaefke B, Yang Q, Wang L, Wang F, Wang L (2022) The subthalamic corticotropin-releasing hormone neurons mediate adaptive REM-sleep responses to threat. Neuron 110(7):1223–1239e1228. https://doi.org/10.1016/j.neuron.2021.12.033
Cai P, Chen HY, Tang WT, Hu YD, Chen SY, Lu JS, Lin ZH, Huang SN, Hu LH, Su WK, Li QX, Lin ZJ, Kang TR, Yan XB, Liu PC, Chen L, Yin D, Wu SY, Li HY, Yu C (2022) A glutamatergic basal forebrain to midbrain circuit mediates wakefulness and defensive behavior. Neuropharmacology 208:108979. https://doi.org/10.1016/j.neuropharm.2022.108979
Scammell TE, Arrigoni E, Lipton JO (2017) Neural circuitry of Wakefulness and Sleep. Neuron 93(4):747–765. https://doi.org/10.1016/j.neuron.2017.01.014
Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J (2011) Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 519(5):933–956. https://doi.org/10.1002/cne.22559
Mohan Kumar V, Mallick BN, Chhina GS, Singh B (1984) Influence of ascending reticular activating system on preoptic neuronal activity. Exp Neurol 86(1):40–52. https://doi.org/10.1016/0014-4886(84)90065-7
Chen CR, Zhong YH, Jiang S, Xu W, Xiao L, Wang Z, Qu WM, Huang ZL (2021) Dysfunctions of the paraventricular hypothalamic nucleus induce hypersomnia in mice. eLife 10. https://doi.org/10.7554/eLife.69909
Ma T, Wong SZH, Lee B, Ming GL, Song H (2021) Decoding neuronal composition and ontogeny of individual hypothalamic nuclei. Neuron 109(7):1150–1167e1156. https://doi.org/10.1016/j.neuron.2021.01.026
Cheung CC, Krause WC, Edwards RH, Yang CF, Shah NM, Hnasko TS, Ingraham HA (2015) Sex-dependent changes in metabolism and behavior, as well as reduced anxiety after eliminating ventromedial hypothalamus excitatory output. Mol metabolism 4(11):857–866. https://doi.org/10.1016/j.molmet.2015.09.001
Herman JP, Tasker JG, Ziegler DR, Cullinan WE (2002) Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacology, biochemistry, and behavior 71. 457–468. https://doi.org/10.1016/s0091-3057(01)00681-5. 3
McClellan KM, Parker KL, Tobet S (2006) Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocr 27(2):193–209. https://doi.org/10.1016/j.yfrne.2006.02.002
Tejas-Juárez JG, Cruz-Martínez AM, López-Alonso VE, García-Iglesias B, Mancilla-Díaz JM, Florán-Garduño B, Escartín-Pérez RE (2014) Stimulation of dopamine D4 receptors in the paraventricular nucleus of the hypothalamus of male rats induces hyperphagia: involvement of glutamate. Physiol Behav 133:272–281. https://doi.org/10.1016/j.physbeh.2014.04.040
Ren S, Wang Y, Yue F, Cheng X, Dang R, Qiao Q, Sun X, Li X, Jiang Q, Yao J, Qin H, Wang G, Liao X, Gao D, Xia J, Zhang J, Hu B, Yan J, Wang Y, Xu M, Han Y, Tang X, Chen X, He C, Hu Z (2018) The paraventricular thalamus is a critical thalamic area for wakefulness. Sci (New York NY) 362(6413):429–434. https://doi.org/10.1126/science.aat2512
Zhang J, Chen D, Sweeney P, Yang Y (2020) An excitatory ventromedial hypothalamus to paraventricular thalamus circuit that suppresses food intake. Nat Commun 11(1):6326. https://doi.org/10.1038/s41467-020-20093-4
Bailey M, Silver R (2014) Sex differences in circadian timing systems: implications for disease. Front Neuroendocr 35(1):111–139. https://doi.org/10.1016/j.yfrne.2013.11.003
Orozco-Solis R, Aguilar-Arnal L, Murakami M, Peruquetti R, Ramadori G, Coppari R, Sassone-Corsi P (2016) The circadian clock in the Ventromedial Hypothalamus Controls Cyclic Energy expenditure. Cell Metabol 23(3):467–478. https://doi.org/10.1016/j.cmet.2016.02.003
Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald AM, Ross SE (2020) Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the Pain response. Neuron 106(6):927–939e925. https://doi.org/10.1016/j.neuron.2020.03.014
Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, Flak J, Neve RL, Evans ML, Lowell BB, Myers MG Jr, Heisler LK (2014) A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metabol 20(6):1030–1037. https://doi.org/10.1016/j.cmet.2014.11.006
Yamamoto R, Ahmed N, Ito T, Gungor NZ, Pare D (2018) Optogenetic Study of Anterior BNST and Basomedial Amygdala Projections to the Ventromedial Hypothalamus. eNeuro 5 (3). https://doi.org/10.1523/eneuro.0204-18.2018
Shao J, Gao DS, Liu YH, Chen SP, Liu N, Zhang L, Zhou XY, Xiao Q, Wang LP, Hu HL, Yang F (2022) Cav3.1-driven bursting firing in ventromedial hypothalamic neurons exerts dual control of anxiety-like behavior and energy expenditure. Mol Psychiatry 27(6):2901–2913. https://doi.org/10.1038/s41380-022-01513-x
Kunwar PS, Zelikowsky M, Remedios R, Cai H, Yilmaz M, Meister M, Anderson DJ (2015) Ventromedial hypothalamic neurons control a defensive emotion state. eLife 4 https://doi.org/10.7554/eLife.06633
Wang L, Chen IZ, Lin D (2015) Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron 85(6):1344–1358. https://doi.org/10.1016/j.neuron.2014.12.025
Wang X, Zhang C, Szábo G, Sun QQ (2013) Distribution of CaMKIIα expression in the brain in vivo, studied by CaMKIIα-GFP mice. Brain Res 1518:9–25. https://doi.org/10.1016/j.brainres.2013.04.042
Frankiensztajn LM, Elliott E, Koren O (2020) The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr Opin Neurobiol 62:76–82. https://doi.org/10.1016/j.conb.2019.12.003
Ono D, Mukai Y, Hung CJ, Chowdhury S, Sugiyama T, Yamanaka A (2020) The mammalian circadian pacemaker regulates wakefulness via CRF neurons in the paraventricular nucleus of the hypothalamus. Sci Adv 6(45). https://doi.org/10.1126/sciadv.abd0384
Liu Y, Rao B, Li S, Zheng N, Wang J, Bi L, Xu H (2022) Distinct hypothalamic paraventricular nucleus inputs to the Cingulate Cortex and Paraventricular Thalamic Nucleus modulate anxiety and Arousal. Front Pharmacol 13:814623. https://doi.org/10.3389/fphar.2022.814623
Anafi RC, Kayser MS, Raizen DM (2019) Exploring phylogeny to find the function of sleep. Nat Rev Neurosci 20(2):109–116. https://doi.org/10.1038/s41583-018-0098-9
Nissen C, Piosczyk H, Holz J, Maier JG, Frase L, Sterr A, Riemann D, Feige B (2021) Sleep is more than rest for plasticity in the human cortex. Sleep 44(3). https://doi.org/10.1093/sleep/zsaa216
Stickgold R (2005) Sleep-dependent memory consolidation. Nature 437(7063):1272–1278. https://doi.org/10.1038/nature04286
Mason GM, Lokhandwala S, Riggins T, Spencer RMC (2021) Sleep and human cognitive development. Sleep Med Rev 57:101472. https://doi.org/10.1016/j.smrv.2021.101472
Pace-Schott EF, Germain A, Milad MR (2015) Effects of sleep on memory for conditioned fear and fear extinction. Psychol Bull 141(4):835–857. https://doi.org/10.1037/bul0000014
Li YD, Luo YJ, Xu W, Ge J, Cherasse Y, Wang YQ, Lazarus M, Qu WM, Huang ZL (2021) Ventral pallidal GABAergic neurons control wakefulness associated with motivation through the ventral tegmental pathway. Mol Psychiatry 26(7):2912–2928. https://doi.org/10.1038/s41380-020-00906-0
Kerman IA, Clinton SM, Simpson DN, Bedrosian TA, Bernard R, Akil H, Watson SJ (2012) Inborn differences in environmental reactivity predict divergent diurnal behavioral, endocrine, and gene expression rhythms. Psychoneuroendocrinology 37(2):256–269. https://doi.org/10.1016/j.psyneuen.2011.06.010
Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA (2007) The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J neuroscience: official J Soc Neurosci 27(50):13624–13634. https://doi.org/10.1523/jneurosci.2858-07.2007
Fremeau RT Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH (2001) The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31(2):247–260. https://doi.org/10.1016/s0896-6273(01)00344-0
van Veen JE, Kammel LG, Bunda PC, Shum M, Reid MS, Massa MG, Arneson D, Park JW, Zhang Z, Joseph AM, Hrncir H, Liesa M, Arnold AP, Yang X, Correa SM (2020) Hypothalamic estrogen receptor alpha establishes a sexually dimorphic regulatory node of energy expenditure. Nat metabolism 2(4):351–363. https://doi.org/10.1038/s42255-020-0189-6
Viskaitis P, Irvine EE, Smith MA, Choudhury AI, Alvarez-Curto E, Glegola JA, Hardy DG, Pedroni SMA, Paiva Pessoa MR, Fernando ABP, Katsouri L, Sardini A, Ungless MA, Milligan G, Withers DJ (2017) Modulation of SF1 neuron activity Coordinately regulates both feeding Behavior and Associated Emotional States. Cell Rep 21(12):3559–3572. https://doi.org/10.1016/j.celrep.2017.11.089
Funding
Declaration.
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Haibo Xu and Yidan Li designed this study. The technical guidance were given by Yue Li, Xuefen Zhang, Ying Li and Yanchao Liu. Yidan li performed the experiments, analyzed the data, and drafted the manuscript. Haibo Xu revised the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yidan Li is the first author.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Li, Y., Zhang, X. et al. CaMKIIa Neurons of the Ventromedial Hypothalamus Mediate Wakefulness and Anxiety-like Behavior. Neurochem Res 48, 2463–2475 (2023). https://doi.org/10.1007/s11064-023-03925-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03925-9