Abstract
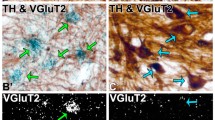
The nucleus accumbens shell is a critical node in reward circuitry, encoding environments associated with reward. Long-range inputs from the ventral hippocampus (ventral subiculum) to the nucleus accumbens shell have been identified, yet their precise molecular phenotype remains to be determined. Here we used retrograde tracing to identify the ventral subiculum as the brain region with the densest glutamatergic (VGluT1–Slc17a7) input to the shell. We then used circuit-directed translating ribosome affinity purification to examine the molecular characteristics of distinct glutamatergic (VGluT1, VGluT2–Slc17a6) ventral subiculum to nucleus accumbens shell projections. We immunoprecipitated translating ribosomes from this population of projection neurons and analysed molecular connectomic information using RNA sequencing. We found differential gene enrichment across both glutamatergic projection neuron subtypes. In VGluT1 projections, we found enrichment of Pfkl, a gene involved in glucose metabolism. In VGluT2 projections, we found a depletion of Sparcl1 and Dlg1, genes known to play a role in depression- and addiction-related behaviours. These findings highlight potential glutamatergic neuronal-projection-specific differences in ventral subiculum to nucleus accumbens shell projections. Together these data advance our understanding of the phenotype of a defined brain circuit.







Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article. For additional information, please contact the corresponding authors.
References
Salgado S, Kaplitt MG (2015) The nucleus accumbens: a comprehensive review. Stereotact Funct Neurosurg 93(2):75–93
Bossert JM et al (2007) Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 27(46):12655–12663
French SJ, Totterdell S (2002) Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol 446(2):151–165
French S, Totterdell S (2003) Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience 119(1):19–31
Böhm C et al (2018) Routes to, from and within the subiculum. Cell Tissue Res 373(3):557–563
Tsao A, Moser M-B, Moser EI (2013) Traces of experience in the lateral entorhinal cortex. Curr Biol 23(5):399–405
Marchant NJ et al (2016) Role of ventral subiculum in context-induced relapse to alcohol seeking after punishment-imposed abstinence. J Neurosci 36(11):3281–3294
Bossert JM et al (2016) Role of projections from ventral subiculum to nucleus accumbens shell in context-induced reinstatement of heroin seeking in rats. Psychopharmacology 233(10):1991–2004
Anne C, Gasnier B (2014) Vesicular neurotransmitter transporters: mechanistic aspects. In: Current topics in membranes. Elsevier, pp 149–174
El Mestikawy S et al (2011) From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci 12(4):204–216
Shigeri Y, Seal RP, Shimamoto K (2004) Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev 45(3):250–265
Fremeau RT Jr et al (2002) The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci 99(22):14488–14493
Schafer MK et al (2002) Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem 277(52):50734–50748
Gras C et al (2002) A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci 22(13):5442–5451
Herzog E et al (2004) Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience 123(4):983–1002
Herzog E et al (2006) Synaptic and vesicular co-localization of the glutamate transporters VGLUT1 and VGLUT2 in the mouse hippocampus. J Neurochem 99(3):1011–1018
Fremeau RT Jr et al (2001) The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31(2):247–260
Varoqui H et al (2002) Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci 22(1):142–155
Jin S et al (2022) Examining ventral subiculum and basolateral amygdala projections to the nucleus accumbens shell: Differential expression of VGLuT1, VGLuT2 and VGaT in the rat. Neurosci Lett 788:136858
Borgius L et al (2010) A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol Cell Neurosci 45(3):245–257
Paxinos G, Franklin KB (2004) The mouse brain in stereotaxic coordinates: compact. Elsevier, Amsterdam
Pazos A, Cortes R, Palacios JM (1985) Thyrotropin-releasing hormone receptor binding sites: autoradiographic distribution in the rat and guinea pig brain. J Neurochem 45:1448–1463
Nectow AR, Ekstrand MI, Friedman JM (2015) Molecular characterization of neuronal cell types based on patterns of projection with Retro-TRAP. Nat Protoc 10(9):1319–1327
Paxinos G, Franklin KB (2004) The mouse brain in stereotaxic coordinates. Gulf Professional Publishing
Heiman M et al (2014) Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP). Nat Protoc 9(6):1282–1291
Ip CK et al (2019) Amygdala NPY circuits promote the development of accelerated obesity under chronic stress conditions. Cell Metab 30(1):111-128.e6
Liao Y, Shi W (2020) Read trimming is not required for mapping and quantification of RNA-seq reads at the gene level. NAR Genomics Bioinform 2(3):lqaa068
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9(4):357–359
Liu Y, Schmidt B (2012) Long read alignment based on maximal exact match seeds. Bioinformatics 28(18):i318–i324
Kim D et al (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14(4):1–13
Anders S, Pyl PT, Huber W (2015) HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31(2):166–169
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):1–21
Krishnan V et al (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131(2):391–404
McCormack K et al (2002) Genetic analysis of the Mammalian K+ channel β subunit Kvβ2 (Kcnab2)∗. J Biol Chem 277(15):13219–13228
Peled-Kamar M et al (1998) Altered brain glucose metabolism in transgenic-PFKL mice with elevated L-phosphofructokinase: in vivo NMR studies. Brain Res 810(1–2):138–145
Block I et al (2019) CFP suppresses breast cancer cell growth by TES-mediated upregulation of the transcription factor DDIT3. Oncogene 38(23):4560–4573
Klingler A et al (2020) Species-, organ-and cell-type-dependent expression of SPARCL1 in human and mouse tissues. PLoS ONE 15(5):e0233422
Nzounza P et al (2012) The scaffolding protein Dlg1 is a negative regulator of cell-free virus infectivity but not of cell-to-cell HIV-1 transmission in T cells. PLoS ONE 7(1):e30130
Zhang Z et al (2011) Fuz regulates craniofacial development through tissue specific responses to signaling factors. PLoS ONE 6(9):e24608
Phillipson O, Griffiths A (1985) The topographic order of inputs to nucleus accumbens in the rat. Neuroscience 16(2):275–296
Groenewegen HJ et al (1999) Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci 877(1):49–63
Ekstrand MI et al (2014) Molecular profiling of neurons based on connectivity. Cell 157(5):1230–1242
Castellano P et al (2016) Methamphetamine compromises gap junctional communication in astrocytes and neurons. J Neurochem 137(4):561–575
Kokarovtseva L et al (2009) Excitability and gap junction–mediated mechanisms in nucleus accumbens regulate self-stimulation reward in rats. Neuroscience 159(4):1257–1263
Li C-Y, Mao X, Wei L (2008) Genes and (common) pathways underlying drug addiction. PLoS Comput Biol 4(1):e2
Blendy JA, Maldonado R (1998) Genetic analysis of drug addiction: the role of cAMP response element binding protein. J Mol Med 76:104–110
Kohnhorst CL et al (2017) Identification of a multienzyme complex for glucose metabolism in living cells. J Biol Chem 292(22):9191–9203
Koponen H et al (2015) Association between suicidal behaviour and impaired glucose metabolism in depressive disorders. BMC Psychiatry 15(1):1–8
Volkow ND et al (1994) Recovery of brain glucose metabolism in detoxified alcoholics: correction
Kukurba KR, Montgomery SB (2015) RNA sequencing and analysis. Cold Spring Harbor Protoc 201(11):pdb-top084970
Ozsolak F, Milos PM (2011) RNA sequencing: advances, challenges and opportunities. Nat Rev Genet 12(2):87–98
Hindson CM et al (2013) Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods 10(10):1003–1005
Harris MJ, Juriloff DM (2010) An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A 88(8):653–669
Ayimugu A et al (2020) Investigation of the involvement of Parkin in Parkinson’s disease and cancer by monitoring the changes in SH-SY5Y cells at the nuclear proteome level. Anticancer Res 40(6):3169–3190
Irie Y et al (2011) Methamphetamine induces endoplasmic reticulum stress related gene CHOP/Gadd153/ddit3 in dopaminergic cells. Cell Tissue Res 345(2):231–241
Maiya R et al (2012) DlgS97/SAP97, a neuronal isoform of discs large, regulates ethanol tolerance. PLoS ONE 7(11):e48967
Vialou V et al (2010) ΔFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 13(6):745–752
Hindson BJ et al (2011) High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 83(22):8604–8610
Wang S, Hodges S, Skvortsow D (2013) Droplet digital PCR: ultra-sensitive validation technology for RNA-seq
Acknowledgements
This project involved several multi-disciplinary and cross-institutional collaborations. We would firstly like to thank Dr. Alexander Nectow and Professor Jeffrey Friedman from The Rockefeller University for providing the pAAV-EF1a-FLEX-NBL10-HA construct and critical methodological information. We acknowledge The Rockefeller University Bi-Institutional Antibody & Bioresource Core Facility at the Memorial Sloan Kettering Cancer Center for providing the Htz GFP monoclonal antibodies for immunoprecipitation. We thank Professor Zane Andrews at Monash University for providing VGluT1- and VGluT2-cre mice. We acknowledge the assistance of Dr. Vicky Perreau, Professor Christine Wells, Samuel Tanner and Franca Casagranda from The University of Melbourne for assistance with initial experimental design, bioinformatic analysis and ddPCR probe design. We thank Kym Pham from The University of Melbourne Center for Cancer Research for assistance with RNA sequencing. Finally, we thank Dr. Fatemeh Zanganeh, Dr. Sarah Ch’ng, Dr. Leigh Walker, Dr. Roberta Anversa, Associate Professor Daniel Scott and Liubov Lee-Kardashyan from the Florey Institute for assistance with experimental procedures, bioinformatic analysis and constructive conceptual input.
Funding
This work was supported by a National Health and Medical Research Council Project Grant to AJL and EJC (1159032) and a National Health and Medical Research Council Synergy Grant to AJL (2009851), a Brain and Behavior Research Foundation NARSAD Young Investigator Grant to EJC (28007) and a University of Melbourne Early Career Researcher Grant to EJC. We acknowledge the Victorian State Government Operational Infrastructure Scheme. This work was also supported in part through the NIH/NCI Cancer Center Support Grant GrantP30 CA008748 which funds The Rockefeller University Bi-Institutional Antibody and Bioresource Core Facility.
Author information
Authors and Affiliations
Contributions
Study conception by AJL. All authors contributed to the study design. Material preparation, data collection and analysis were performed by SJ, EJC, CKI and SL. The first draft of the manuscript was written by EJC, SJ and AJL. All authors edited and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
All authors report no competing interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, S., Campbell, E.J., Ip, C.K. et al. Molecular Profiling of VGluT1 AND VGluT2 Ventral Subiculum to Nucleus Accumbens Shell Projections. Neurochem Res 48, 2490–2501 (2023). https://doi.org/10.1007/s11064-023-03921-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03921-z