Abstract
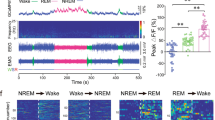
The ventral pallidum (VP) is involved in the regulation of a variety of behaviors such as motor, reward, and behavioral motivation, and the ability to perform these functions properly is dependent on a high degree of wakefulness. It is unknown whether VP CaMKIIa-expression (VPCaMKIIa) neurons also have a role in sleep–wake regulation and related neuronal circuit mechanisms. In the present experiment, we first used in vivo fiber photometry to find the population activity of VPCaMKIIa neurons which increased during the transitions from non-rapid-eye movement (NREM) sleep to wakefulness and NREM sleep to rapid-eye-movement (REM) sleep, with decreased during the transitions from wakefulness to NREM sleep. Then chemogenetic activation of VPCaMKIIa neurons induced an increase in wakefulness that lasted for 2 h. Mice that were exposed to short-term optogenetic stimulation woke up quickly from stable NREM sleep, and long-term optogenetic stimulation maintained wakefulness. In addition, optogenetic activation of the axons of VPCaMKIIa neurons in the lateral habenula (LHb) also facilitated the initiation and maintenance of wakefulness and mediated anxiety-like behavior. Finally, the method of chemogenetic inhibition was employed to suppress VPCaMKIIa neurons, and yet, inhibition of VPCaMKIIa neuronal activity did not result in an increase in NREM sleep and a decrease in wakefulness. Overall, our data illustrate that the activation of VPCaMKIIa neurons is of great importance for promoting wakefulness.





Similar content being viewed by others
Data Availability
Data supporting the results of this study are available from the corresponding author.
References
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW (2012) Control of sleep and wakefulness. Physiol Rev 92(3):1087–1187. https://doi.org/10.1152/physrev.00032.2011
Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH (1988) Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci 8(11):4007–4026. https://doi.org/10.1523/jneurosci.08-11-04007.1988
Cape EG, Jones BE (2000) Effects of glutamate agonist versus procaine microinjections into the basal forebrain cholinergic cell area upon gamma and theta EEG activity and sleep-wake state. Eur J Neurosci 12(6):2166–2184. https://doi.org/10.1046/j.1460-9568.2000.00099.x
Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J (2011) Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 519(5):933–956. https://doi.org/10.1002/cne.22559
Irmak SO, de Lecea L (2014) Basal forebrain cholinergic modulation of sleep transitions. Sleep 37(12):1941–1951. https://doi.org/10.5665/sleep.4246
Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang WC, Weissbourd B, Sakai N, Luo L, Nishino S, Dan Y (2015) Basal forebrain circuit for sleep-wake control. Nat Neurosci 18(11):1641–1647. https://doi.org/10.1038/nn.4143
Mogenson G, Jones D, Yim C (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14(2–3):69–97. https://doi.org/10.1016/0301-0082(80)90018-0
Gong W, Neill DB, Lynn M, Justice JB (1999) Dopamine D1/D2 agonists injected into nucleus accumbens and ventral pallidum differentially affect locomotor activity depending on site. Neuroscience 93(4):1349–1358. https://doi.org/10.1016/s0306-4522(99)00235-3
Graves SM, Viskniskki AA, Cunningham KA, Napier TC (2013) Serotonin(2C) receptors in the ventral pallidum regulate motor function in rats. NeuroReport 24(11):605–608. https://doi.org/10.1097/WNR.0b013e3283630af5
Farrar AM, Font L, Pereira M, Mingote S, Bunce JG, Chrobak JJ, Salamone JD (2008) Forebrain circuitry involved in effort-related choice: injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience 152(2):321–330. https://doi.org/10.1016/j.neuroscience.2007.12.034
Ottenheimer D, Richard JM, Janak PH (2018) Ventral pallidum encodes relative reward value earlier and more robustly than nucleus accumbens. Nat Commun 9(1):4350. https://doi.org/10.1038/s41467-018-06849-z
Richard JM, Ambroggi F, Janak PH, Fields HL (2016) Ventral pallidum neurons encode incentive value and promote cue-elicited instrumental actions. Neuron 90(6):1165–1173. https://doi.org/10.1016/j.neuron.2016.04.037
Smith KS, Berridge KC (2005) The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci 25(38):8637–8649. https://doi.org/10.1523/JNEUROSCI.1902-05.2005
Smith KS, Tindell AJ, Aldridge JW, Berridge KC (2009) Ventral pallidum roles in reward and motivation. Behav Brain Res 196(2):155–167. https://doi.org/10.1016/j.bbr.2008.09.038
Root DH, Melendez RI, Zaborszky L, Napier TC (2015) The ventral pallidum: subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol 130:29–70. https://doi.org/10.1016/j.pneurobio.2015.03.005
Saga Y, Richard A, Sgambato-Faure V, Hoshi E, Tobler PN, Tremblay L (2017) Ventral pallidum encodes contextual information and controls aversive behaviors. Cereb Cortex 27(4):2528–2543. https://doi.org/10.1093/cercor/bhw107
Kaplan A, Mizrahi-Kliger AD, Israel Z, Adler A, Bergman H (2020) Dissociable roles of ventral pallidum neurons in the basal ganglia reinforcement learning network. Nat Neurosci 23(4):556–564. https://doi.org/10.1038/s41593-020-0605-y
Lenard L, Ollmann T, Laszlo K, Kovacs A, Galosi R, Kallai V, Attila T, Kertes E, Zagoracz O, Karadi Z, Peczely L (2017) Role of D2 dopamine receptors of the ventral pallidum in inhibitory avoidance learning. Behav Brain Res 321:99–105. https://doi.org/10.1016/j.bbr.2017.01.005
Faget L, Zell V, Souter E, McPherson A, Ressler R, Gutierrez-Reed N, Yoo JH, Dulcis D, Hnasko TS (2018) Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nat Commun 9(1):849. https://doi.org/10.1038/s41467-018-03125-y
Hjelmstad GO, Xia Y, Margolis EB, Fields HL (2013) Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. J Neurosci 33(15):6454–6459. https://doi.org/10.1523/JNEUROSCI.0178-13.2013
Knowland D, Lilascharoen V, Pacia CP, Shin S, Wang EH, Lim BK (2017) Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell 170(2):284-297 e218. https://doi.org/10.1016/j.cell.2017.06.015
Herrera CG, Cadavieco MC, Jego S, Ponomarenko A, Korotkova T, Adamantidis A (2016) Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat Neurosci 19(2):290–298. https://doi.org/10.1038/nn.4209
Venner A, Anaclet C, Broadhurst RY, Saper CB, Fuller PM (2016) A novel population of wake-promoting GABAergic neurons in the ventral lateral hypothalamus. Curr Biol 26(16):2137–2143. https://doi.org/10.1016/j.cub.2016.05.078
Wang RF, Guo H, Jiang SY, Liu ZL, Qu WM, Huang ZL, Wang L (2021) Control of wakefulness by lateral hypothalamic glutamatergic neurons in male mice. J Neurosci Res 99(6):1689–1703. https://doi.org/10.1002/jnr.24828
Yang SR, Hu ZZ, Luo YJ, Zhao YN, Sun HX, Yin D, Wang CY, Yan YD, Wang DR, Yuan XS, Ye CB, Guo W, Qu WM, Cherasse Y, Lazarus M, Ding YQ, Huang ZL (2018) The rostromedial tegmental nucleus is essential for non-rapid eye movement sleep. PLoS Biol. 16(4):e2002909. https://doi.org/10.1371/journal.pbio.2002909
Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L (2016) VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci 19(10):1356–1366. https://doi.org/10.1038/nn.4377
Yu X, Li W, Ma Y, Tossell K, Harris JJ, Harding EC, Ba W, Miracca G, Wang D, Li L, Guo J, Chen M, Li Y, Yustos R, Vyssotski AL, Burdakov D, Yang Q, Dong H, Franks NP, Wisden W (2019) GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci 22(1):106–119. https://doi.org/10.1038/s41593-018-0288-9
Zhang YP, Holbro N, Oertner TG (2008) Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Natl Acad Sci U S A 105(33):12039–12044. https://doi.org/10.1073/pnas.0802940105
Stephenson-Jones M, Bravo-Rivera C, Ahrens S, Furlan A, Xiao X, Fernandes-Henriques C, Li B (2020) Opposing contributions of GABAergic and glutamatergic ventral pallidal neurons to motivational behaviors. Neuron 105(5):921-933 e925. https://doi.org/10.1016/j.neuron.2019.12.006
Liu B, Cao Y, Wang J, Dong J (2020) Excitatory transmission from ventral pallidum to lateral habenula mediates depression. World J Biol Psychiatry 21(8):627–633. https://doi.org/10.1080/15622975.2020.1725117
Zuo W, Zuo Q, Wu L, Mei Q, Shah M, Zheng J, Li D, Xu Y, Ye JH (2021) Roles of corticotropin-releasing factor signaling in the lateral habenula in anxiety-like and alcohol drinking behaviors in male rats. Neurobiol Stress 15:100395. https://doi.org/10.1016/j.ynstr.2021.100395
Luo YJ, Li YD, Wang L, Yang SR, Yuan XS, Wang J, Cherasse Y, Lazarus M, Chen JF, Qu WM, Huang ZL (2018) Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors. Nat Commun 9(1):1576. https://doi.org/10.1038/s41467-018-03889-3
Oishi Y, Xu Q, Wang L, Zhang BJ, Takahashi K, Takata Y, Luo YJ, Cherasse Y, Schiffmann SN, de Kerchove DA, Urade Y, Qu WM, Huang ZL, Lazarus M (2017) Slow-wave sleep is controlled by a subset of nucleus accumbens core neurons in mice. Nat Commun 8(1):734. https://doi.org/10.1038/s41467-017-00781-4
Zhang X, Liu Y, Yang B, Xu H (2020) Inactivation of the ventral pallidum by GABAA receptor agonist promotes non-rapid eye movement sleep in rats. Neurochem Res 45(8):1791–1801. https://doi.org/10.1007/s11064-020-03040-z
Li YD, Luo YJ, Xu W, Ge J, Cherasse Y, Wang YQ, Lazarus M, Qu WM, Huang ZL (2021) Ventral pallidal GABAergic neurons control wakefulness associated with motivation through the ventral tegmental pathway. Mol Psychiatry 26(7):2912–2928. https://doi.org/10.1038/s41380-020-00906-0
Kroeger D, Ferrari LL, Petit G, Mahoney CE, Fuller PM, Arrigoni E, Scammell TE (2017) Cholinergic, glutamatergic, and GABAergic neurons of the pedunculopontine tegmental nucleus have distinct effects on sleep/wake behavior in mice. J Neurosci 37(5):1352–1366. https://doi.org/10.1523/JNEUROSCI.1405-16.2016
Tsunematsu T, Kilduff TS, Boyden ES, Takahashi S, Tominaga M, Yamanaka A (2011) Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci 31(29):10529–10539. https://doi.org/10.1523/JNEUROSCI.0784-11.2011
Saper CB, Scammell TE, Lu J (2005) Hypothalamic regulation of sleep and circadian rhythms. Nature 437(7063):1257–1263. https://doi.org/10.1038/nature04284
Scammell TE, Arrigoni E, Lipton JO (2017) Neural circuitry of wakefulness and sleep. Neuron 93(4):747–765. https://doi.org/10.1016/j.neuron.2017.01.014
Borbely A (2022) The two-process model of sleep regulation: beginnings and outlook. J Sleep Res 31(4):e13598. https://doi.org/10.1111/jsr.13598
Hu H, Cui Y, Yang Y (2020) Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci 21(5):277–295. https://doi.org/10.1038/s41583-020-0292-4
Guilding C, Hughes ATL, Piggins HD (2010) Circadian oscillators in the epithalamus. Neuroscience 169(4):1630–1639. https://doi.org/10.1016/j.neuroscience.2010.06.015
Paul MJ, Indic P, Schwartz WJ (2011) A role for the habenula in the regulation of locomotor activity cycles. Eur J Neurosci 34(3):478–488. https://doi.org/10.1111/j.1460-9568.2011.07762.x
Zhao H, Rusak B (2005) Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience 132(2):519–528. https://doi.org/10.1016/j.neuroscience.2005.01.012
Liu C, Liu J, Zhou L, He H, Zhang Y, Cai S, Yuan C, Luo T, Zheng J, Yu T, Zhang M (2021) Lateral habenula glutamatergic neurons modulate isoflurane anesthesia in mice. Front Mol Neurosci 14:628996. https://doi.org/10.3389/fnmol.2021.628996
Jacinto LR, Mata R, Novais A, Marques F, Sousa N (2017) The habenula as a critical node in chronic stress-related anxiety. Exp Neurol 289:46–54. https://doi.org/10.1016/j.expneurol.2016.12.003
Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP (2008) Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One 3(1):e1506. https://doi.org/10.1523/JNEUROSCI.18-01-00411.1998
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
YL and HX: designed this research. YL: wrote the first draft of manuscript and performed animal studies. YL and YL: performed the behaviour test. XZ: performed the immunohistochemistry experiments. Data collection and analysis were performed by all authors. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Zhang, X., Li, Y. et al. Activation of Ventral Pallidum CaMKIIa-Expressing Neurons Promotes Wakefulness. Neurochem Res 48, 2502–2513 (2023). https://doi.org/10.1007/s11064-023-03915-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03915-x