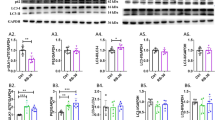
Abstract
Activating transcription factor 6 (ATF6) is an endoplasmic reticulum (ER) stress-regulated transcription factor that induces expression of major molecular chaperones in the ER. We recently reported that ATF6β, a subtype of ATF6, promoted survival of hippocampal neurons exposed to ER stress and excitotoxicity, at least in part by inducing expression of calreticulin, an ER molecular chaperone with high Ca2+-binding capacity. In the present study, we demonstrate that ATF6β deficiency in mice also decreases calreticulin expression and increases expression of glucose-regulated protein 78, another ER molecular chaperone, in emotional brain regions such as the prefrontal cortex (PFC), hypothalamus, hippocampus, and amygdala. Comprehensive behavioral analyses revealed that Atf6b−/− mice exhibit anxiety-like behavior in the light/dark transition test and hyperactivity in the forced swim test. Consistent with these results, PFC and hypothalamic corticotropin-releasing hormone (CRH) expression was increased in Atf6b−/− mice, as was circulating corticosterone. Moreover, CRH receptor 1 antagonism alleviated anxiety-like behavior in Atf6b−/− mice. These findings suggest that ATF6β deficiency produces anxiety-like behavior and hyperactivity via a CRH receptor 1-dependent mechanism. ATF6β could play a role in psychiatric conditions in the emotional centers of the brain.





Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125:443–451. https://doi.org/10.1016/j.cell.2006.04.014
Mori K (2009) Signalling pathways in the unfolded protein response: development from yeast to mammals. J Biochem 146:743–750. https://doi.org/10.1093/jb/mvp166
Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L et al (2007) Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci 27:901–908. https://doi.org/10.1523/JNEUROSCI.4289-06.2007
Sprencke NT, Sims SG, Sánchez CL, Meares GP (2017) Endoplasmic reticulum stress and inflammation in the central nervous system. Mol Neurodegener 12:42. https://doi.org/10.1186/s13024-017-0183-y
Thiebaut AM, Hedou E, Marciniak SJ, Vivien D, Roussel BD (2019) Proteostasis during cerebral ischemia. Front Neurosci 13:637. https://doi.org/10.3389/fnins.2019.00637
Hayashi T, Suet TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate ca(2+) signaling and cell survival. Cell 131:596–610. https://doi.org/10.1016/j.cell.2007.08.036
Nguyen LD, Fischer TT, Abreu D, Arroyo A, Urano F, Ehrlich BE (2020) Calpain inhibitor and ibudilast rescue β cell functions in a cellular model of Wolfram syndrome. Proc Natl Acad Sci USA 117:17389–17398. https://doi.org/10.1073/pnas.2007136117
Kato T, Ishiwata M, Yamada K, Kasahara T, Kakiuchi C, Iwamoto K et al (2008) Behavioral and gene expression analyses of Wfs1 knockout mice as a possible animal model of mood disorder. Neurosci Res 61:143–158. https://doi.org/10.1016/j.neures.2008.02.002
Luuk H, Plaas M, Raud S, Innos J, Sütt S, Lasner H et al (2009) Wfs1-deficient mice display impaired behavioural adaptation in stressful environment. Behav Brain Res 198:334–345. https://doi.org/10.1016/j.bbr.2008.11.007
Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP (2009) Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res 198:472–476. https://doi.org/10.1016/j.bbr.2008.11.036
Martínez G, Vidal RL, Mardones P, Serrano FG, Ardiles AO, Wirth C et al (2016) Regulation of memory formation by the transcription factor XBP1. Cell Rep 14:1382–1394. https://doi.org/10.1016/j.celrep.2016.01.028
Haze K, Yoshida H, Yanagi H, Yura T, Mori K (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10:3787–3799. https://doi.org/10.1091/mbc.10.11.3787
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086. https://doi.org/10.1126/science.1209038
Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R et al (2000) ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6:1355–1364. https://doi.org/10.1016/s1097-2765(00)00133-7
Hashida K, Kitao Y, Sudo H, Awa Y, Maeda S, Mori K et al (2012) ATF6alpha promotes astroglial activation and neuronal survival in a chronic mouse model of Parkinson’s disease. PLoS ONE 7:e47950. https://doi.org/10.1371/journal.pone.0047950
Yoshikawa A, Kamide T, Hashida K, Ta HM, Inahata Y, Takarada-Iemata M et al (2015) Deletion of Atf6α impairs astroglial activation and enhances neuronal death following brain ischemia in mice. J Neurochem 132:342–353. https://doi.org/10.1111/jnc.12981
Kezuka D, Tkarada-Iemata M, Hattori T, Mori K, Takahashi R, Kitao Y et al (2016) Deletion of Atf6alpha enhances kainate-induced neuronal death in mice. Neurochem Int 92:67–74. https://doi.org/10.1016/j.neuint.2015.12.009
Ta HM, Le TM, Ishii H, Takarada-Iemata M, Hattori T, Hashida K et al (2016) Atf6α deficiency suppresses microglial activation and ameliorates pathology of experimental autoimmune encephalomyelitis. J Neurochem 139:1124–1137. https://doi.org/10.1111/jnc.13714
Nguyen DT, Le TM, Hattori T, Takarada-Iemata M, Ishii H, Roboon J et al (2021) The ATF6β-calreticulin axis promotes neuronal survival under endoplasmic reticulum stress and excitotoxicity. Sci Rep 11:13086. https://doi.org/10.1038/s41598-021-92529-w
Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H et al (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13:365–376. https://doi.org/10.1016/j.devcel.2007.07.018
Hattori T, Shimizu S, Koyama Y, Emoto H, Matsumoto Y, Kumamoto N et al (2014) DISC1 (disrupted-in-schizophrenia-1) regulates differentiation of oligodendrocytes. PLoS ONE 9:e88506. https://doi.org/10.1371/journal.pone.0088506
Miyakawa T, Yamada M, Duttaroy A, Wess J (2001) Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci 21:5239–5250. https://doi.org/10.1523/JNEUROSCI.21-14-05239.2001
Takao K, Miyakawa T (2006) Light/dark transition test for mice. J Vis Exp 13:104. https://doi.org/10.3791/104
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33. https://doi.org/10.1016/s0014-2999(03)01272-x
Komada M, Takao K, Miyakawa T (2008) Elevated plus maze for mice. J Vis Exp 22:1088. https://doi.org/10.3791/1088
Omata Y, Aoki R, Aoki-Yoshida A, Hiemori K, Toyoda A, Tateno H et al (2018) Reduced fucosylation in the distal intestinal epithelium of mice subjected to chronic social defeat stress. Sci Rep 8:13199. https://doi.org/10.1038/s41598-018-31403-8
Shoji H, Takao K, Hattori S, Miyakawa T (2016) Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain 9:11. https://doi.org/10.1186/s13041-016-0191-9
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336
Fujioka R, Nii T, Iwaki A, Shibata A, Ito I, Kitaichi K et al (2014) Comprehensive behavioral study of mGluR3 knockout mice: implication in schizophrenia related endophenotypes. Mol Brain 7:31. https://doi.org/10.1186/1756-6606-7-31
McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP et al (2015) CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87:605–620. https://doi.org/10.1016/j.neuron.2015.07.002
Tanaka T, Shimizu S, Ueno M, Fujihara Y, Ikawa M, Miyata S (2018) MARCKSL1 regulates spine formation in the amygdala and controls the hypothalamic-pituitary-adrenal axis and anxiety-like behaviors. EBioMedicine 30:62–73. https://doi.org/10.1016/j.ebiom.2018.03.018
de Kloet ER, Joëls M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475. https://doi.org/10.1038/nrn1683
Herman JP, Tasker JG (2016) Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol 7:137. https://doi.org/10.3389/fendo.2016.00137
Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O et al (2007) CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446:41–45. https://doi.org/10.1038/nature05526
Terauchi A, Johnson-Venkatesh EM, Bullock B, Lehtinen MK, Umemori H (2016) Retrograde fibroblast growth factor 22 (FGF22) signaling regulates insulin-like growth factor 2 (IGF2) expression for activity-dependent synapse stabilization in the mammalian brain. Elife 5:e12151. https://doi.org/10.7554/eLife.12151
Khan S (2019) IGFBP-2 signaling in the brain: from brain development to higher order brain functions. Front Endocrinol 10:822. https://doi.org/10.3389/fendo.2019.00822
Farivar R, Zangenehpour S, Chaudhuri A (2004) Cellular-resolution activity mapping of the brain using immediate-early gene expression. Front Biosci 9:104–109. https://doi.org/10.2741/1198
Binder EB (2009) The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 34:186–195. https://doi.org/10.1016/j.psyneuen.2009.05.021
Ke X, Fu Q, Majnik A, Cohen S, Liu Q, Lane R (2018) Adverse early life environment induces anxiety-like behavior and increases expression of FKBP5 mRNA splice variants in mouse brain. Physiol Genomics 50:973–981. https://doi.org/10.1152/physiolgenomics.00054.2018
Riad MH, Park K, Ibañez-Tallon I, Heintz N (2022) Local production of corticotropin-releasing hormone in prefrontal cortex modulates male-specific novelty exploration. Proc Natl Acad Sci USA 119:e2211454119. https://doi.org/10.1073/pnas.2211454119
Acknowledgements
We thank S. Muramoto (Kanazawa Medical University) for support with the experiments. We would also like to thank Drs. Y. Ishigaki, H. Nishizono, and Y. Kaneko (Animal Resources and Genetic Engineering Center, Kanazawa Medical University) for technical assistance with Atf6b−/− mice.
Funding
This work was supported in part by JSPS KAKENHI (Grant Numbers JP17K13079 and JP20K11222) and by AMED (Grant Number JP20gm1410005s0301).
Author information
Authors and Affiliations
Contributions
Takashi Tanaka conceived the project, designed and performed all behavioral tests and ELISAs, analyzed the data, and wrote the manuscript. Dinh Thi Nguyen, Nichakarn Kwankaew, Hiroshi Ishii, Mika Takarada-Iemata, and Tsuyoshi Hattori performed in situ hybridization, RT-qPCR studies, and immunohistochemistry experiments. Megumi Sumizono conducted statistical analyses. Reika Shinoda performed genotyping of Atf6b−/− mice. Nobuo Kato supported experiments. Seiichi Oyadomari and Kazutoshi Mori provided Atf6b−/− mice. Osamu Hori searched the literature, wrote the manuscript, and supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanaka, T., Nguyen, D.T., Kwankaew, N. et al. ATF6β Deficiency Elicits Anxiety-like Behavior and Hyperactivity Under Stress Conditions. Neurochem Res 48, 2175–2186 (2023). https://doi.org/10.1007/s11064-023-03900-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03900-4