Abstract
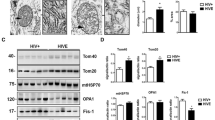
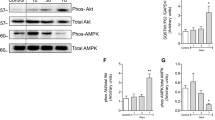
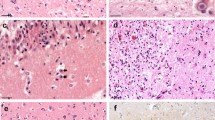
Mitochondria dysfunction may be an important contributor to Japanese encephalitis (JE) viral infection disease pathogenesis. In the current study, we define whether changes in mitochondrial DNA copy number (which is the biomarker for mitochondrial function) and alteration in mitochondria dynamics (fusion and fission) contribute to the pathology of the JE virus in vivo mice model. We found decreased mitochondria copy number, reduced activation of mitochondrial fission (FIS1/DRP1), and increased activation of mitochondrial fusion (MFN1/MFN2/OPA1) genes that are associated with increased NOX2-mediated ROS generation and neuronal cell death following JE virus infection. Furthermore, we found that antioxidant glutathione level decreases. In summary, the following study demonstrates that JE viral infection causes an imbalance in mitochondrial fission/fusion gene activation and promotes NOX2-mediated oxidative stress and cell death, suggesting that intervention in mitochondrial dynamics might be a potential therapeutic strategy for combating oxidative stress and inflammatory process in JE viral infection.



Similar content being viewed by others
References
Kumar A, Barrett JP, Alvarez-Croda DM et al (2016) NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain Behav Immun 58:291–309. https://doi.org/10.1016/J.BBI.2016.07.158
Singh S, Singh G, Tiwari S, Kumar A (2020) CCR2 inhibition reduces neurotoxic microglia activation phenotype after japanese encephalitis viral infection. Front Cell Neurosci 14. https://doi.org/10.3389/FNCEL.2020.00230/FULL
Bratic A, Larsson NG (2013) The role of mitochondria in aging.Journal of Clinical Investigation123
Currais A (2015) Ageing and inflammation - A central role for mitochondria in brain health and disease.Ageing Res Rev21
van Horssen J, van Schaik P, Witte M (2019) Inflammation and mitochondrial dysfunction: a vicious circle in neurodegenerative disorders?Neurosci Lett710
López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN (2013) Mitochondrial dysfunction and the inflammatory response.Mitochondrion13
Pawelec G, Goldeck D, Derhovanessian E (2014) Inflammation, ageing and chronic disease.Curr Opin Immunol29
Mengel-From J, Thinggaard M, Dalgård C et al (2014) Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet 133. https://doi.org/10.1007/s00439-014-1458-9
Knez J, Winckelmans E, Plusquin M et al (2016) Correlates of Peripheral Blood mitochondrial DNA content in a General Population. Am J Epidemiol 183. https://doi.org/10.1093/aje/kwv175
Nicolson GL (2014) Mitochondrial Dysfunction and Chronic Disease: Treatment With Natural Supplements. Integrative Medicine: A Clinician’s Journal 13:35
Kornfeld OS, Hwang S, Disatnik MH et al (2015) Mitochondrial reactive oxygen species at the heart of the matter: New therapeutic approaches for cardiovascular diseases.Circ Res116
Zhang K, Kaufman RJ (2008) From endoplasmic-reticulum stress to the inflammatory response.Nature454
Frank S (2006) Dysregulation of mitochondrial fusion and fission: an emerging concept in neurodegeneration.Acta Neuropathol111
Skulachev VP (2001) Mitochondrial filaments and clusters as intracellular power-transmitting cables.Trends Biochem Sci26
Bereiter-Hahn J, Vöth M (1994) Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech 27:198–219. https://doi.org/10.1002/JEMT.1070270303
Santel A, Fuller MT (2001) Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114:867–874. https://doi.org/10.1242/JCS.114.5.867
Stiles L, Shirihai OS (2012) Mitochondrial Dynamics and morphology in Beta-cells. Best Pract Res Clin Endocrinol Metab 26:725. https://doi.org/10.1016/J.BEEM.2012.05.004
Wai T, Langer T (2016) Mitochondrial Dynamics and metabolic regulation. Trends Endocrinol Metab 27:105–117. https://doi.org/10.1016/J.TEM.2015.12.001
Ikeda Y, Shirakabe A, Brady C et al (2015) Molecular mechanisms mediating mitochondrial dynamics and mitophagy and their functional roles in the cardiovascular system. J Mol Cell Cardiol 78:116–122. https://doi.org/10.1016/J.YJMCC.2014.09.019
Li J, Wang Y, Wang Y et al (2015) Pharmacological activation of AMPK prevents Drp1-mediated mitochondrial fission and alleviates endoplasmic reticulum stress-associated endothelial dysfunction. J Mol Cell Cardiol 86. https://doi.org/10.1016/j.yjmcc.2015.07.010
Li Y, Yang J, Chen MH et al (2015) Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol Res 99. https://doi.org/10.1016/j.phrs.2015.05.012
Shukla V, Shakya AK, Shukla M et al (2016) Circulating levels of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases during japanese encephalitis virus infection. Virusdisease 27:63–76. https://doi.org/10.1007/S13337-015-0301-9
Chiu HP, Chiu H, Yang CF et al (2018) Inhibition of japanese encephalitis virus infection by the host zinc-finger antiviral protein. PLoS Pathog 14. https://doi.org/10.1371/JOURNAL.PPAT.1007166
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protocols 2008 3(6):1101–1108. https://doi.org/10.1038/nprot.2008.73
Augustyniak J, Lenart J, Zychowicz M et al (2017) Mitochondrial biogenesis and neural differentiation of human iPSC is modulated by idebenone in a developmental stage-dependent manner. Biogerontology 18:665–677. https://doi.org/10.1007/S10522-017-9718-4
Fuke S, Kubota-Sakashita M, Kasahara T et al (2011) Regional variation in mitochondrial DNA copy number in mouse brain. Biochim et Biophys Acta (BBA) - Bioenergetics 1807:270–274. https://doi.org/10.1016/J.BBABIO.2010.11.016
Abdel-Salam OME, Abdel-Rahman RF, Sleem AA, Farrag AR (2012) Modulation of lipopolysaccharide-induced oxidative stress by capsaicin. Inflammopharmacology 20:207–217. https://doi.org/10.1007/S10787-011-0101-9
Niknahad H, Heidari R, Mohammadzadeh R et al (2017) Sulfasalazine induces mitochondrial dysfunction and renal injury. Ren Fail 39:745. https://doi.org/10.1080/0886022X.2017.1399908
Seljeskog E, Hervig T, Mansoor MA (2006) A novel HPLC method for the measurement of thiobarbituric acid reactive substances (TBARS). A comparison with a commercially available kit. Clin Biochem 39:947–954. https://doi.org/10.1016/J.CLINBIOCHEM.2006.03.012
Barrett JP, Henry RJ, Villapol S et al (2017) NOX2 deficiency alters macrophage phenotype through an IL-10/STAT3 dependent mechanism: implications for traumatic brain injury. J Neuroinflammation 14. https://doi.org/10.1186/S12974-017-0843-4
Fukai T, Ushio-Fukai M (2020) Cross-talk between NADPH oxidase and Mitochondria: role in ROS Signaling and Angiogenesis. Cells 9. https://doi.org/10.3390/CELLS9081849
Zorov DB, Filburn CR, Klotz LO et al (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192:1001–1014. https://doi.org/10.1084/JEM.192.7.1001
Zinkevich NS, Gutterman DD (2011) ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol 301. https://doi.org/10.1152/AJPHEART.01271.2010
Randow F, MacMicking JD, James LC (2013) Cellular Self-Defense: how Cell-Autonomous Immunity protects against pathogens. Science 340:701–706. https://doi.org/10.1126/SCIENCE.1233028
Liao SL, Raung SL, Chen CJ (2002) Japanese encephalitis virus stimulates superoxide dismutase activity in rat glial cultures. Neurosci Lett 324:133–136. https://doi.org/10.1016/S0304-3940(02)00236-7
Go YM, Jones DP (2008) Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta 1780:1273–1290. https://doi.org/10.1016/J.BBAGEN.2008.01.011
Srivastava R, Kalita J, Khan MY, Misra UK (2009) Free radical generation by neurons in rat model of japanese encephalitis. Neurochem Res 34:2141–2146. https://doi.org/10.1007/S11064-009-0008-7
Kumar S, Misra UK, Kalita J et al (2009) Imbalance in oxidant/antioxidant system in different brain regions of rat after the infection of japanese encephalitis virus. Neurochem Int 55:648–654. https://doi.org/10.1016/J.NEUINT.2009.06.008
Tian Y, Jiang W, Gao N et al (2010) Inhibitory effects of glutathione on dengue virus production. Biochem Biophys Res Commun 397:420–424. https://doi.org/10.1016/J.BBRC.2010.05.108
Kumar A, Kalita J, Sinha RA et al (2020) Impaired Autophagy Flux is Associated with Proinflammatory Microglia Activation following japanese encephalitis virus infection. Neurochem Res 45:2184–2195. https://doi.org/10.1007/S11064-020-03080-5
Yang TC, Lai CC, Shiu SL et al (2010) Japanese encephalitis virus down-regulates thioredoxin and induces ROS-mediated ASK1-ERK/p38 MAPK activation in human promonocyte cells. Microbes Infect 12:643–651. https://doi.org/10.1016/J.MICINF.2010.04.007
Kalita J, Misra UK, Pandey S, Dhole TN (2003) A comparison of clinical and radiological findings in adults and children with japanese encephalitis. Arch Neurol 60:1760–1764. https://doi.org/10.1001/ARCHNEUR.60.12.1760
Sprenger HG, Langer T (2019) The good and the Bad of mitochondrial breakups. Trends Cell Biol 29:888–900. https://doi.org/10.1016/J.TCB.2019.08.003
Youle RJ, van der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337:1062–1065. https://doi.org/10.1126/SCIENCE.1219855
Ren L, Chen X, Chen X et al (2020) Mitochondrial Dynamics: Fission and Fusion in Fate determination of mesenchymal stem cells. Front Cell Dev Biol 8. https://doi.org/10.3389/FCELL.2020.580070
Chen H, Chan DC (2005) Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet 14 Spec No 2. https://doi.org/10.1093/HMG/DDI270
Cassidy-Stone A, Chipuk JE, Ingerman E et al (2008) Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 14:193–204. https://doi.org/10.1016/J.DEVCEL.2007.11.019
Rappold PM, Cui M, Grima JC et al (2014) Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat Commun 5. https://doi.org/10.1038/NCOMMS6244
Grohm J, Kim SW, Mamrak U et al (2012) Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death & Differentiation 2012 19:9 19:1446–1458. https://doi.org/10.1038/cdd.2012.18
Zhao YX, Cui M, Chen SF et al (2014) Amelioration of ischemic mitochondrial Injury and bax-dependent outer membrane permeabilization by Mdivi‐1. CNS Neurosci Ther 20:528. https://doi.org/10.1111/CNS.12266
Li Y, Zhou ZH, Chen MH et al (2016) Inhibition of mitochondrial fission and NOX2 expression prevent NLRP3 inflammasome activation in the endothelium: the role of Corosolic Acid Action in the amelioration of endothelial dysfunction. Antioxid Redox Signal 24:893–908. https://doi.org/10.1089/ARS.2015.6479
van den Bossche J, Baardman J, Otto NA et al (2016) Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep 17:684–696. https://doi.org/10.1016/J.CELREP.2016.09.008
Kumar A, Kalita J, Sinha RA et al (2020) Impaired Autophagy Flux is Associated with Proinflammatory Microglia Activation following japanese encephalitis virus infection. Neurochemical Res 2020 45:9. https://doi.org/10.1007/S11064-020-03080-5
Wei H, Liu L, Chen Q (2015) Selective removal of mitochondria via mitophagy: distinct pathways for different mitochondrial stresses. Biochimica et Biophysica Acta (BBA) -. Mol Cell Res 1853:2784–2790. https://doi.org/10.1016/J.BBAMCR.2015.03.013
Rodriguez JL, Costlow JL, Sheedy M, Yoon KT, Gabaldón AM, Steel JJ (2022) Sindbis Virus Replication reduces dependence on mitochondrial metabolism during infection. Front Cell Infect Microbiol 16:12:859814
Addabbo F, Montagnani M, Goligorsky MS (2009) Mitochondria and reactive oxygen species. Hypertension 53:885–892
Fujimoto M, Hayashi T (2011) New Insights into the role of Mitochondria-Associated endoplasmic reticulum membrane. Int Rev Cell Mol Biol 292:73–117
Keck F, Brooks-Faulconer T, Lark T, Ravishankar P, Bailey C, Salvador-Morales C et al (2017) Altered mitochondrial Dynamics as a consequence of venezuelan equine encephalitis virus infection. Virulence 8:1849–1866
Khan M, Syed GH, Kim S-J, Siddiqui A (2015) Mitochondrial Dynamics and viral infections: a close Nexus. Biochim. Biophys. Acta (BBA) - Mol. Cell Res 1853:2822–2833
Acknowledgements
None.
Funding
The following study was supported by the Ramalingaswami fellowship (BT/RLF/Re-entry/13/2014) from the Department of Biotechnology, Ministry of Science and Technology, Govt. of India and Grant-in-aid Scheme of the Department of Health Research (DHR-GIA) (R.11,013/66/2021-GIA/HR) to AK.
Author information
Authors and Affiliations
Contributions
AK, GS: Conceptualization, Formal analysis, Investigation, Validation, Visualization, Writing an original draft, Methodology, Data curation. Both authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, G., Kumar, A. Japanese Encephalitis Virus Infection Causes an Imbalance in the Activation of Mitochondrial Fusion/Fission Genes and Triggers the Activation of NOX2-mediated Oxidative Stress and Neuronal Cell Death. Neurochem Res 48, 2196–2205 (2023). https://doi.org/10.1007/s11064-023-03898-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03898-9