Abstract
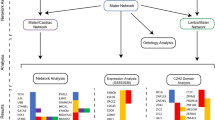
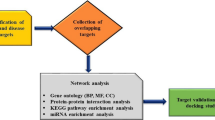
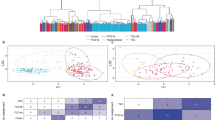
Chemotherapeutic agents such as methotrexate (MTX), raltitrexed (RTX), 5-fluorouracil (5-FU), hydroxyurea (HU), and retinoic acid (RA), and valproic acid (VPA), an antiepileptic drug, all can cause malformations in the developing central nervous system (CNS), such as neural tube defects (NTDs). However, the common pathogenic mechanisms remain unclear. This study aimed to explore the mechanisms of NTDs caused by MTX, RTX, 5-FU, HU, RA, and VPA (MRFHRV), based on network pharmacology and molecular biology experiments. The MRFHRV targets were integrated with disease targets, to find the potential molecules related to MRFHRV-induced NTDs. Protein–protein interaction analysis and molecular docking were performed to analyze these common targets. Utilizing the kyoto encyclopedia of genes and genomes (KEGG) signaling pathways, we analyzed and searched the possible causative pathogenic mechanisms by crucial targets and the signaling pathway. Results showed that MRFHRV induced NTDs through several key targets (including TP53, MAPK1, HSP90AA1, ESR1, GRB2, HDAC1, EGFR, PIK3CA, RXRA, and FYN) and multiple signaling pathways such as PI3K/Akt pathway, suggesting that abnormal proliferation and differentiation could be critical pathogenic contributors in NTDs induced by MRFHRV. These results were further validated by CCK8 assay in mouse embryonic stem cells and GFAP staining in embryonic brain tissue. This study indicated that chemotherapeutic and antiepileptic agents induced NTDs might through predicted targets TP53, MAPK1, GRB2, HDAC1, EGFR, PIK3CA, RXRA, and FYN and multiple signaling pathways. More caution was required for the clinical administration for women with childbearing potential and pregnant.









Similar content being viewed by others
Data availability
The data used to support the finding of this study are available from the corresponding author upon request.
References
de Haan J, Verheecke M, Van Calsteren K, Van Calster B, Shmakov RG, Gziri MM, Halaska MJ, Fruscio R, Lok CA, Boere IA (2018) Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol 19:337–346
Lee J-M, Yan P, Xiao Q, Chen S, Lee K-Y, Hsu CY, Xu J (2008) Methylprednisolone protects oligodendrocytes but not neurons after spinal cord injury. J Neurosci 28:3141–3149
Şanlı AM, Serbes G, Sargon MF, Çalışkan M, Kılınç K, Bulut H, Şekerci Z (2012) Methothrexate attenuates early neutrophil infiltration and the associated lipid peroxidation in the injured spinal cord but does not induce neurotoxicity in the uninjured spinal cord in rats. Acta Neurochir 154:1045–1054
Sulpher J, Dattilo F, Dent S, Turek M, Reaume MN, Johnson C (2014) Acute cardiogenic shock induced by infusional 5-fluorouracil. Case Rep Oncol Med 2014:1–3
Cunningham D, Zalcberg J, Maroun J, James R, Clarke S, Maughan T, Vincent M, Schulz J, Barón MG, Facchini T (2002) Efficacy, tolerability and management of raltitrexed (Tomudex™) monotherapy in patients with advanced colorectal cancer: A review of phase II/III trials. Eur J Cancer 38:478–486
Pennell PB, French JA, Harden CL, Davis A, Bagiella E, Andreopoulos E, Lau C, Llewellyn N, Barnard S, Allien S (2018) Fertility and birth outcomes in women with epilepsy seeking pregnancy. JAMA Neurol 75:962–969
Lu R-B, Chang Y-H, Lee S-Y, Wang T-Y, Cheng S-L, Chen P-S, Yang Y-K, Hong J-S, Chen S-L (2020) Dextromethorphan protect the valproic acid induced downregulation of neutrophils in patients with bipolar disorder. Clinic Psychopharmacol Neurosci 18:145
Collins MD, Mao GE (1999) Teratology of retinoids. Annu Rev Pharmacol Toxicol 39:399–430
Skalko RG, Gold MP (1974) Teratogenicity of methotrexate in mice. Teratology 9:159–163
Wilson KS, Malfair Taylor SC (2009) Raltitrexed: optimism and reality. Expert Opin Drug Metab Toxicol 5:1447–1454
Dagg C, Schlager G, Doerr A (1966) Polygenic control of the teratogenicity of 5-fluorouracil in mice. Genetics 53:1101
Wilson JG, Scott WJ, Ritter EJ, Fradkin R (1975) Comparative distribution and embryotoxicity of hydroxyurea in pregnant rats and rhesus monkeys. Teratology 11:169–178
Błaszczyk B, Miziak B, Pluta R, Czuczwar SJ (2022) Epilepsy in pregnancy—management principles and focus on valproate. Int J Mol Sci 23:1369
Al-Saleh E, Al-Harmi J, Al-Rashdan I, Al-Shammari M, Nandakumaran M (2007) Maternal–fetal transport kinetics of methotrexate in perfused human placenta: In vitro study. J Matern Fetal Neonatal Med 20:411–418
Boike GM, Deppe G, Young JD, Malone JM Jr, Malviya VK, Sokol RJ (1989) Chemotherapy in a pregnant rat model: 2.5-Fluorouracil: Nonlinear kinetics and placental transfer. Gynecol Oncol 34:191–194
Semczuk-Sikora A, Czuczwar S, Semczuk A, Kwasniewska A, Semczuk M (2010) Valproic acid transfer across human placental cotyledon during dual perfusion in vitro. Ann Agric Environ Med 17:153–157
Liu D, Xue J, Liu Y, Gu H, Wei X, Ma W, Luo W, Ma L, Jia S, Dong N (2018) Inhibition of NRF2 signaling and increased reactive oxygen species during embryogenesis in a rat model of retinoic acid-induced neural tube defects. Neurotoxicology 69:84–92
Wei X, Ma W, Gu H, Liu D, Luo W, Bai Y, Wang W, Lui VCH, Yang P, Yuan Z (2020) Transamniotic mesenchymal stem cell therapy for neural tube defects preserves neural function through lesion-specific engraftment and regeneration. Cell Death Dis 11:1–16
Shan L, Fan Y, Li H, Liu W, Gu H, Zhou F, Yuan Z (2012) Proteomic analysis of amniotic fluid of pregnant rats with spina bifida aperta. J Proteomics 75:1181–1189
An D, Wei X, Li H, Gu H, Huang T, Zhao G, Liu B, Wang W, Chen L, Ma W (2015) Identification of PCSK9 as a novel serum biomarker for the prenatal diagnosis of neural tube defects using iTRAQ quantitative proteomics. Sci Rep 5:1–11
Zhao J, Guan T, Wang J, Xiang Q, Wang M, Wang X, Guan Z, Xie Q, Niu B, Zhang T (2013) Influence of the antifolate drug Methotrexate on the development of murine neural tube defects and genomic instability. J Appl Toxicol 33:915–923
Dong Y, Wang X, Zhang J, Guan Z, Xu L, Wang J, Zhang T, Niu B (2015) Raltitrexed’s effect on the development of neural tube defects in mice is associated with DNA damage, apoptosis, and proliferation. Mol Cell Biochem 398:223–231
Wang X, Guan Z, Dong Y, Zhu Z, Wang J, Niu B (2018) Inhibition of thymidylate synthase affects neural tube development in mice. Reprod Toxicol 76:17–25
Guan Z, Wang X, Dong Y, Xu L, Zhu Z, Wang J, Zhang T, Niu B (2015) dNTP deficiency induced by HU via inhibiting ribonucleotide reductase affects neural tube development. Toxicology 328:142–151
Zhao L, Liu D, Ma W, Gu H, Wei X, Luo W, Yuan Z (2021) Bhlhe40/Sirt1 axis-regulated mitophagy is implicated in all-trans retinoic acid-induced spina bifida aperta. Fronti Cell Develop Biol 9:1025
Steele JW, Lin YL, Chen N, Wlodarczyk BJ, Chen Q, Attarwala N, Venkatesalu M, Cabrera RM, Gross SS, Finnell RH (2022) Embryonic hypotaurine levels contribute to strain-dependent susceptibility in mouse models of valproate-induced neural tube defects. Fronti Cell Develop Biol. https://doi.org/10.3389/fcell.2022.832492
Bhandari J, Thada PK (2021) Neural tube disorders. In: StatPearls [Internet]. StatPearls Publishing
Okano H (2002) Stem cell biology of the central nervous system. J Neurosci Res 69:698–707
Hsu Y-C, Lee D-C, Chiu I-M (2007) Neural stem cells, neural progenitors, and neurotrophic factors. Cell Transplant 16:133–150
Finkel Z, Esteban F, Rodriguez B, Fu T, Ai X, Cai L (2021) Diversity of adult neural stem and progenitor cells in physiology and disease. Cells 10:2045
Chen N, Xu J, Zhang X, Li S, Zhu W, Cui H, Sun Y, Han B, Ma A (2021) Effect of Notch1 on neural tube defects and neural stem cell differentiation induced by all-trans retinoic acid. Mol Med Rep 23:1–1
Lowery LA, Sive H (2004) Strategies of vertebrate neurulation and a re-evaluation of teleost neural tube formation. Mech Dev 121:1189–1197
Nikolopoulou E, Galea GL, Rolo A, Greene ND, Copp AJ (2017) Neural tube closure: cellular, molecular and biomechanical mechanisms. Development 144:552–566
Oh KK, Adnan M, Cho DH (2020) Network pharmacology of bioactives from Sorghum bicolor with targets related to diabetes mellitus. PLoS ONE 15:e0240873
Oh KK, Adnan M, Cho DH (2021) Active ingredients and mechanisms of Phellinus linteus (grown on Rosa multiflora) for alleviation of Type 2 diabetes mellitus through network pharmacology. Gene 768:145320
Hähnke VD, Kim S, Bolton EE (2018) PubChem chemical structure standardization. Journal of cheminformatics 10:1–40
Gfeller D, Grosdidier A, Wirth M, Daina A, Michielin O, Zoete V (2014) SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res 42:W32–W38
Consortium U (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47:D506–D515
Ray HJ, Niswander LA (2016) Grainyhead-like 2 downstream targets act to suppress epithelial-to-mesenchymal transition during neural tube closure. Development 143:1192–1204
Tian T, Lai X, Xiang K, Han X, Yin S, Cabrera RM, Steele JW, Lei Y, Cao X, Finnell RH (2022) Hypermethylation of PI3K-AKT signalling pathway genes is associated with human neural tube defects. Epigenetics 17:133–146
Yu M, Li W, Luo S, Zhang Y, Liu H, Gao Y, Wang X, Wilson JX, Huang G (2014) Folic acid stimulation of neural stem cell proliferation is associated with altered methylation profile of PI3K/Akt/CREB. J Nutr Biochem 25:496–502
Fan G, Martinowich K, Chin MH, He F, Fouse SD, Hutnick L, Hattori D, Ge W, Shen Y, Wu H (2005) DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development 132:3345
Guris DL, Fantes J, Tara D, Druker BJ, Imamoto A (2001) Mice lacking the homologue of the human 22q11. 2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nat Genet 27:293–298
Rodier PM (1995) Developing brain as a target of toxicity. Environ Health Perspect 103:73–76
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108:511–533
Robaey P, Krajinovic M, Marcoux S, Moghrabi A (2008) Pharmacogenetics of the neurodevelopmental impact of anticancer chemotherapy. Dev Disabil Res Rev 14:211–220
Ouzir M, El Bairi K, Amzazi S (2016) Toxicological properties of fenugreek (Trigonella foenum graecum). Food Chem Toxicol 96:145–154
Feiock C, Yagi M, Maidman A, Rendahl A, Hui S, Seelig D (2016) Central nervous system injury–a newly observed bystander effect of radiation. PLoS ONE 11:e0163233
Yang M, Kim J-S, Kim J, Jang S, Kim S-H, Kim J-C, Shin T, Wang H, Moon C (2012) Acute treatment with methotrexate induces hippocampal dysfunction in a mouse model of breast cancer. Brain Res Bull 89:50–56
Yamada S, Yamazaki D, Kanda Y (2018) 5-Fluorouracil inhibits neural differentiation via Mfn1/2 reduction in human induced pluripotent stem cells. J Toxicol Sci 43:727–734
Zhao H, Wang Q, Yan T, Zhang Y, Xu H-j, Yu H-p, Tu Z, Guo X, Jiang Y-h, Li X-j (2019) Maternal valproic acid exposure leads to neurogenesis defects and autism-like behaviors in non-human primates. Transl Psychiatry 9:1–13
Szutowicz A, Bielarczyk H, Jankowska-Kulawy A, Ronowska A, Pawełczyk T (2015) Retinoic acid as a therapeutic option in Alzheimer’s disease: a focus on cholinergic restoration. Expert Rev Neurother 15:239–249
Pennimpede T, Cameron DA, MacLean GA, Li H, Abu-Abed S, Petkovich M (2010) The role of CYP26 enzymes in defining appropriate retinoic acid exposure during embryogenesis. Birth Defects Res A 88:883–894
Neary J, Norenberg L, Norenberg M (1988) Protein kinase C in primary astrocyte cultures: cytoplasmic localization and translocation by a phorbol ester. J Neurochem 50:1179–1184
Fedoroff S, Vernadakis A (1986) Astrocytes: Biochemistry, physiology, and pharmacology of astrocytes. Academic Press
Ruutiainen J, Newcombe J, Salmi A, Dahl D, Frey H (1981) Measurement of glial fibrillary acidic protein (GFAP) and anti-GFAP antibodies by solid-phase radioimmunoassays. Acta Neurol Scand 63:297–305
Bovolenta P, Liem RK, Mason CA (1984) Development of cerebellar astroglia: transitions in form and cytoskeletal content. Dev Biol 102:248–259
Sun X, Jiang R, Zhang Y, Chen M, Xiang P, Qi Y, Gao Q, Huang B, Ge J (2009) Gene expression and differentiation characteristics in mice E13.5 and E17.5 neural retinal progenitors. Mol Vis 15:2503
Kessaris N, Pringle N, Richardson WD (2008) Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Phil Trans Royal Soc B 363:71–85
Oria M, Figueira RL, Scorletti F, Sbragia L, Owens K, Li Z, Pathak B, Corona MU, Marotta M, Encinas JL (2018) CD200-CD200R imbalance correlates with microglia and pro-inflammatory activation in rat spinal cords exposed to amniotic fluid in retinoic acid-induced spina bifida. Sci Rep 8:1–12
Oria M, Pathak B, Li Z, Bakri K, Gouwens K, Varela MF, Lampe K, Murphy KP, Lin C-Y, Peiro JL (2022) Premature neural progenitor cell differentiation into astrocytes in retinoic acid-induced spina bifida rat model. Fronti Mol Neurosci. https://doi.org/10.3389/fnmol.2022.888351
Yang S-L, Yang M, Herrlinger S, Liang C, Lai F, Chen J-F (2015) MiR-302/367 regulate neural progenitor proliferation, differentiation timing, and survival in neurulation. Dev Biol 408:140–150
Zhong W, Jiang M-M, Schonemann MD, Meneses JJ, Pedersen RA, Jan LY, Jan YN (2000) Mouse numb is an essential gene involved in cortical neurogenesis. Proc Natl Acad Sci 97:6844–6849
Gottlieb E, Haffner R, King A, Asher G, Gruss P, Lonai P, Oren M (1997) Transgenic mouse model for studying the transcriptional activity of the p53 protein: age-and tissue-dependent changes in radiation-induced activation during embryogenesis. EMBO J 16:1381–1390
Komarova EA, Chernov MV, Franks R, Wang K, Armin G, Zelnick CR, Chin DM, Bacus SS, Stark GR, Gudkov AV (1997) Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J 16:1391–1400
Louis JM, McFarland VW, May P, Mora PT (1988) The phosphoprotein p53 is down-regulated post-transcriptionally during embryogenesis in vertebrates. Biochem Biophys Acta 950:395–402
Schmid P, Lorenz A, Hameister H, Montenarh M (1991) Expression of p53 during mouse embryogenesis. Development 113:857–865
Armesilla-Díaz A, Bragado P, Del Valle I, Cuevas E, Lázaro I, Martin C, Cigudosa J, Silva A (2009) p53 regulates the self-renewal and differentiation of neural precursors. Neuroscience 158:1378–1389
Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, Saitta SC, Landreth GE (2008) Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J Neurosci 28:6983–6995
Ménard C, Hein P, Paquin A, Savelson A, Yang XM, Lederfein D, Barnabé-Heider F, Mir AA, Sterneck E, Peterson AC (2002) An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron 36:597–610
Conti L, De Fraja C, Gulisano M, Migliaccio E, Govoni S, Cattaneo E (1997) Expression and activation of SH2/PTB-containing ShcA adaptor protein reflects the pattern of neurogenesis in the mammalian brain. Proc Natl Acad Sci 94:8185–8190
Shengkai D, Qianqian L (2022) The Effects and Regulatory Mechanism of Flavonoids from Stems and Leaves of Scutellaria baicalensis Georgi in Promoting Neurogenesis and Improving Memory Impairment Mediated by the BDNF-ERK-CREB Signaling Pathway in Rats. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders)
Liu H, Wu H, Wang Y, Wang Y, Wu X, Ju S, Wang X (2012) Inhibition of class II histone deacetylase blocks proliferation and promotes neuronal differentiation of the embryonic rat neural progenitor cells. Acta Neurobiol Exp (Wars) 72:365–376
Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, Van der Kooy D (1996) In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci 16:2649–2658
Schwindt TT, Motta FL, Gabriela FB, Cristina GM, Guimarães AO, Calcagnotto ME, Pesquero JB, Mello LE (2009) Effects of FGF-2 and EGF removal on the differentiationof mouse neural precursor cells. An Acad Bras Ciênc 81:443–452
Schrage K, Koopmans G, Joosten EA, Mey J (2006) Macrophages and neurons are targets of retinoic acid signaling after spinal cord contusion injury. Eur J Neurosci 23:285–295
Mey J (2006) New therapeutic target for CNS injury? The role of retinoic acid signaling after nerve lesions. J Neurobiol 66:757–779
Wahane SD, Hellbach N, Prentzell MT, Weise SC, Vezzali R, Kreutz C, Timmer J, Krieglstein K, Thedieck K, Vogel T (2014) PI3K-p110-alpha-subtype signalling mediates survival, proliferation and neurogenesis of cortical progenitor cells via activation of mTORC 2. J Neurochem 130:255–267
Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV (1999) Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol 145:1209–1218
Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, McMorris FA (2001) A unique role for Fyn in CNS myelination. J Neurosci 21:2039–2047
Wang B, Zhang Y, Dong H, Gong S, Wei B, Luo M, Wang H, Wu X, Liu W, Xu X (2018) Loss of Tctn3 causes neuronal apoptosis and neural tube defects in mice. Cell Death Dis 9:1–12
Wilson NR, Olm-Shipman AJ, Acevedo DS, Palaniyandi K, Hall EG, Kosa E, Stumpff KM, Smith GJ, Pitstick L, Liao EC (2016) SPECC1L deficiency results in increased adherens junction stability and reduced cranial neural crest cell delamination. Sci Rep 6:1–15
Otaegi G, Yusta-Boyo MJ, Vergaño-Vera E, Méndez-Gómez HR, Carrera AC, Abad JL, González M, De la Rosa EJ, Vicario-Abejón C, de Pablo F (2006) Modulation of the PI 3-kinase–Akt signalling pathway by IGF-I and PTEN regulates the differentiation of neural stem/precursor cells. J Cell Sci 119:2739–2748
Han J, Wang B, Xiao Z, Gao Y, Zhao Y, Zhang J, Chen B, Wang X, Dai J (2008) Mammalian target of rapamycin (mTOR) is involved in the neuronal differentiation of neural progenitors induced by insulin. Mol Cell Neurosci 39:118–124
Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H (2006) PTEN negatively regulates neural stem cell self-renewal by modulating G0–G1 cell cycle entry. Proc Natl Acad Sci 103:111–116
Paling NR, Wheadon H, Bone HK, Welham MJ (2004) Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem 279:48063–48070
Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T (2006) Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 25:2697–2707
Acknowledgements
We appreciate the help offered by Prof. Jing Pan during the process. This study was supported by Beijing Natural Science Foundation (No.7222016), National Natural Science Foundation of China (No. 81700777); Research Foundation of Capital Institute of Pediatrics (CXYJ-2021-03).
Funding
National Natural Science Foundation of China, No. 81700777, Beijing Municipal Natural Science Foundation, No.7222016, Research Foundation of Capital Institute of Pediatrics, CXYJ-2021-03.
Author information
Authors and Affiliations
Contributions
Conceptualization: ZG, YL, XW, BN, JW. Data curation: ZG, JW. Formal analysis: ZG, YL, XW, ZZ, JY. Funding acquisition: ZG, JW. Methodology: ZG, YL. Supervision: JW. Writing – original draft: ZG, YL. Writing – review & editing: ZG, YL, XW, JW, BN, SL, ZZ, AY, JY.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal experiment procedures were approved by the Animal Ethics Committee of Capital Institute of Pediatrics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guan, Z., Liang, Y., Wang, X. et al. Unraveling the Mechanisms of Clinical Drugs-Induced Neural Tube Defects Based on Network Pharmacology and Molecular Docking Analysis. Neurochem Res 47, 3709–3722 (2022). https://doi.org/10.1007/s11064-022-03717-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03717-7