Abstract
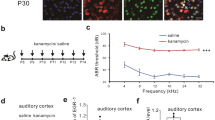
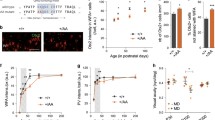
The family of epidermal growth factor (EGF) including neuregulin-1 are implicated in the neuropathology of schizophrenia. We established a rat model of schizophrenia by exposing perinatal rats to EGF and reported that the auditory pathophysiological traits of this model such as prepulse inhibition, auditory steady-state response, and mismatch negativity are relevant to those of schizophrenia. We assessed the activation status of the auditory cortex in this model, as well as that in patients with schizophrenia, by monitoring the three neural activity-induced proteins: EGR1 (zif268), c-fos, and Arc. Among the activity markers, protein levels of EGR1 were significantly higher at the adult stage in EGF model rats than those in control rats. The group difference was observed despite an EGF model rat and a control rat being housed together, ruling out the contribution of rat vocalization effects. These changes in EGR1 levels were seen to be specific to the auditory cortex of this model. The increase in EGR1 levels were detectable at the juvenile stage and continued until old ages but displayed a peak immediately after puberty, whereas c-fos and Arc levels were nearly indistinguishable between groups at all ages with an exception of Arc decrease at the juvenile stage. A similar increase in EGR1 levels was observed in the postmortem superior temporal cortex of patients with schizophrenia. The commonality of the EGR1 increase indicates that the EGR1 elevation in the auditory cortex might be one of the molecular signatures of this animal model and schizophrenia associating with hallucination.




Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Sommer IE, Slotema CW, Daskalakis ZJ, Derks EM, Blom JD, van der Gaag M (2012) The treatment of hallucinations in schizophrenia spectrum disorders. Schizophr Bull 38:704–714. https://doi.org/10.1093/schbul/sbs034
Upthegrove R, Broome MR, Caldwell K, Ives J, Oyebode F, Wood SJ (2016) Understanding auditory verbal hallucinations: a systematic review of current evidence. Acta Psychiatr Scand 133:352–367. https://doi.org/10.1111/acps.12531
Tsang A, Bucci S, Branitsky A, Kaptan S, Rafiq S, Wong S, Berry K, Varese F (2021) The relationship between appraisals of voices (auditory verbal hallucinations) and distress in voice-hearers with schizophrenia-spectrum diagnoses: a meta-analytic review. Schizophr Res 230:38–47. https://doi.org/10.1016/j.schres.2021.02.013
Catani M, Craig MC, Forkel SJ, Kanaan R, Picchioni M, Toulopoulou T, Shergill S, Williams S, Murphy DG, McGuire P (2011) Altered integrity of Perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biol Psychiatry 70:1143–1150. https://doi.org/10.1016/j.biopsych.2011.06.013
Chen C, Wang GH, Wu SH, Zou JL, Zhou Y, Wang HL (2020) Abnormal local activity and functional dysconnectivity in patients with schizophrenia having auditory verbal hallucinations. Curr Med Sci 40:979–984. https://doi.org/10.1007/s11596-020-2271-4
Ropohl A, Sperling W, Elstner S, Tomandl B, Reulbach U, Kaltenhäuser M, Kornhuber J, Maihöfner C (2004) Cortical activity associated with auditory hallucinations. NeuroReport 15:523–526. https://doi.org/10.1097/00001756-200403010-00028
Kasai K, Okazawa K, Nakagome K, Hiramatsu K, Hata A, Fukuda M, Honda M, Miyauchi M, Matsushita M (1999) Mismatch negativity and N2b attenuation as an indicator for dysfunction of the preattentive and controlled processing for deviance detection in schizophrenia: a topographic event-related potential study. Schizophr Res 35:141–156. https://doi.org/10.1016/s0920-9964(98)00116-9
de Weijer AD, Mandl RC, Diederen KM, Neggers SF, Kahn RS, Hulshoff Pol HE, Sommer IE (2011) Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr Res 130:68–77. https://doi.org/10.1016/j.schres.2011.05.010
Wolf ND, Sambataro F, Vasic N, Frasch K, Schmid M, Schönfeldt-Lecuona C, Thomann PA, Wolf RC (2011) Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J Psychiatry Neurosci 36:366–374. https://doi.org/10.1503/jpn.110008
Psomiades M, Fonteneau C, Mondino M, Luck D, Haesebaert F, Suaud-Chagny MF, Brunelin J (2016) Integrity of the arcuate fasciculus in patients with schizophrenia with auditory verbal hallucinations: a DTI-tractography study. Neuroimage Clin 12:970–975. https://doi.org/10.1016/j.nicl.2016.04.013
Woodruff PW, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SC, Shapleske J, Rossell S, David AS, McGuire PK, Murray RM (1997) Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry 154:1676–1682. https://doi.org/10.1176/ajp.154.12.1676
Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK (2000) Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 57:1033–1038. https://doi.org/10.1001/archpsyc.57.11.1033
Lennox BR, Park SB, Medley I, Morris PG, Jones PB (2000) The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res 100:13–20. https://doi.org/10.1016/s0925-4927(00)00068-8
Seeman MV (2021) History of the dopamine hypothesis of antipsychotic action. World J Psychiatry 11:355–364. https://doi.org/10.5498/wjp.v11.i7.355
Schmidt MJ, Mirnics K (2015) Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology 40:190–206. https://doi.org/10.1038/npp.2014.95
Weinberger DR (1996) On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology 14:1s–11s. https://doi.org/10.1016/0893-133x(95)00199-n
Futamura T, Toyooka K, Iritani S, Niizato K, Nakamura R, Tsuchiya K, Someya T, Kakita A, Takahashi H, Nawa H (2002) Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol Psychiatry 7:673–682. https://doi.org/10.1038/sj.mp.4001081
Anttila S, Illi A, Kampman O, Mattila KM, Lehtimäki T, Leinonen E (2004) Association of EGF polymorphism with schizophrenia in Finnish men. NeuroReport 15:1215–1218. https://doi.org/10.1097/00001756-200405190-00027
Groenestege WM, Thébault S, van der Wijst J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van Cutsem E, Hoenderop JG, Knoers NV, Bindels RJ (2007) Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Investig 117:2260–2267. https://doi.org/10.1172/jci31680
Nawa H, Sotoyama H, Iwakura Y, Takei N, Namba H (2014) Neuropathologic implication of peripheral neuregulin-1 and EGF signals in dopaminergic dysfunction and behavioral deficits relevant to schizophrenia: their target cells and time window. Biomed Res Int 2014:697935. https://doi.org/10.1155/2014/697935
Namba H, Nagano T, Jodo E, Eifuku S, Horie M, Takebayashi H, Iwakura Y, Sotoyama H, Takei N, Nawa H (2017) Epidermal growth factor signals attenuate phenotypic and functional development of neocortical GABA neurons. J Neurochem 142:886–900. https://doi.org/10.1111/jnc.14097
Namba H, Nawa H (2020) Post-pubertal difference in nigral dopaminergic cells firing in the schizophrenia model prepared by perinatal challenges of a cytokine, EGF. Neuroscience 441:22–32. https://doi.org/10.1016/j.neuroscience.2020.06.003
Sotoyama H, Namba H, Kobayashi Y, Hasegawa T, Watanabe D, Nakatsukasa E, Sakimura K, Furuyashiki T, Nawa H (2021) Resting-state dopaminergic cell firing in the ventral tegmental area negatively regulates affiliative social interactions in a developmental animal model of schizophrenia. Transl Psychiatry 11:236. https://doi.org/10.1038/s41398-021-01346-2
Jodo E, Inaba H, Narihara I, Sotoyama H, Kitayama E, Yabe H, Namba H, Eifuku S, Nawa H (2019) Neonatal exposure to an inflammatory cytokine, epidermal growth factor, results in the deficits of mismatch negativity in rats. Sci Rep 9:7503. https://doi.org/10.1038/s41598-019-43923-y
Narihara I, Kitajo K, Namba H, Sotoyama H, Inaba H, Watanabe D, Nawa H (2021) Rat call-evoked electrocorticographic responses and intercortical phase synchrony impaired in a cytokine-induced animal model for schizophrenia. Neurosci Res. https://doi.org/10.1016/j.neures.2021.10.007
Joo JY, Schaukowitch K, Farbiak L, Kilaru G, Kim TK (2016) Stimulus-specific combinatorial functionality of neuronal c-fos enhancers. Nat Neurosci 19:75–83. https://doi.org/10.1038/nn.4170
Clements KM, Wainwright PE (2010) Swim stress increases hippocampal Zif268 expression in the spontaneously hypertensive rat. Brain Res Bull 82:259–263. https://doi.org/10.1016/j.brainresbull.2010.05.002
Haines D (2014) Internal morphology of the brain in unstained slices and MRI (9th edition). Lippincott Williams & Wilkins, Philadelphia
Iwakura Y, Wang R, Inamura N, Araki K, Higashiyama S, Takei N, Nawa H (2017) Glutamate-dependent ectodomain shedding of neuregulin-1 type II precursors in rat forebrain neurons. PLoS ONE 12:e0174780. https://doi.org/10.1371/journal.pone.0174780
Iwakura Y, Wang R, Abe Y, Piao YS, Shishido Y, Higashiyama S, Takei N, Nawa H (2011) Dopamine-dependent ectodomain shedding and release of epidermal growth factor in developing striatum: target-derived neurotrophic signaling (Part 2). J Neurochem 118:57–68. https://doi.org/10.1111/j.1471-4159.2011.07295
Brudzynski SM (2005) Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet 35:85–92. https://doi.org/10.1007/s10519-004-0858-3
Gallo FT, Katche C, Morici JF, Medina JH, Weisstaub NV (2018) Immediate early genes, memory and psychiatric disorders: focus on c-Fos, Egr1 and Arc. Front Behav Neurosci 12:79. https://doi.org/10.3389/fnbeh.2018.00079
Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M, Natsume R, Chowdhury S, Sakimura K, Worley PF, Bito H (2012) Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIβ. Cell 149:886–898. https://doi.org/10.1016/j.cell.2012.02.062
Mikuni T, Uesaka N, Okuno H, Hirai H, Deisseroth K, Bito H, Kano M (2013) Arc/Arg3.1 is a postsynaptic mediator of activity-dependent synapse elimination in the developing cerebellum. Neuron 78:1024–1035. https://doi.org/10.1016/j.neuron.2013.04.036
Guzowski JF, Setlow B, Wagner EK, McGaugh JL (2001) Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci 21:5089–5098. https://doi.org/10.1523/jneurosci.21-14-05089.2001
Slattery DA, Morrow JA, Hudson AL, Hill DR, Nutt DJ, Henry B (2005) Comparison of alterations in c-fos and Egr-1 (zif268) expression throughout the rat brain following acute administration of different classes of antidepressant compounds. Neuropsychopharmacology 30:1278–1287. https://doi.org/10.1038/sj.npp.1300717
Lonergan ME, Gafford GM, Jarome TJ, Helmstetter FJ (2010) Time-dependent expression of Arc and zif268 after acquisition of fear conditioning. Neural Plast 2010:139891. https://doi.org/10.1155/2010/139891
Eguchi M, Yamaguchi S (2009) In vivo and in vitro visualization of gene expression dynamics over extensive areas of the brain. Neuroimage 44:1274–1283. https://doi.org/10.1016/j.neuroimage.2008.10.046
Aydin-Abidin S, Trippe J, Funke K, Eysel UT, Benali A (2008) High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp Brain Res 188:249–261. https://doi.org/10.1007/s00221-008-1356-2
Gonzales BJ, Mukherjee D, Ashwal-Fluss R, Loewenstein Y, Citri A (2020) Subregion-specific rules govern the distribution of neuronal immediate-early gene induction. Proc Natl Acad Sci USA 117:23304–23310. https://doi.org/10.1073/pnas.1913658116
Sun A, Lin Y (2016) Npas4: linking neuronal activity to memory. Trends Neurosci 39(4):264–275. https://doi.org/10.1016/j.tins.2016.02.003
Shepard R, Heslin K, Hagerdorn P, Coutellier L (2019) Downregulation of Npas4 in parvalbumin interneurons and cognitive deficits after neonatal NMDA receptor blockade: relevance for schizophrenia. Transl Psychiatry 9(1):99. https://doi.org/10.1038/s41398-019-0436-3
Worley PF, Christy BA, Nakabeppu Y, Bhat RV, Cole AJ, Baraban JM (1991) Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proc Natl Acad Sci USA 88:5106–5110. https://doi.org/10.1073/pnas.88.12.5106
Beckmann AM, Davidson MS, Goodenough S, Wilce PA (1997) Differential expression of Egr-1-like DNA-binding activities in the naive rat brain and after excitatory stimulation. J Neurochem 69:2227–2237. https://doi.org/10.1046/j.1471-4159.1997.69062227.x
Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ (1995) Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64:477–505. https://doi.org/10.1016/0306-4522(94)00355-9
Wisden W, Errington ML, Williams S, Dunnett SB, Waters C, Hitchcock D, Evan G, Bliss TV, Hunt SP (1990) Differential expression of immediate early genes in the hippocampus and spinal cord. Neuron 4:603–614. https://doi.org/10.1016/0896-6273(90)90118-y
Ivkovic S, Kanazir S, Rakic L, Ehrlich ME, Ruzdijic S (1997) Enhanced serum response element binding activity correlates with down-regulation of c-fos mRNA expression in the rat brain following repeated cortical lesions. Brain Res Mol Brain Res 52:62–70. https://doi.org/10.1016/s0169-328x(97)00222-2
Barry DN, Coogan AN, Commins N (2016) The time course of systems consolidation of spatial memory from recent to remote retention: a comparison of the Immediate early genes Zif268, c-Fos and Arc. Neurobiol Learn Mem 128:46–55. https://doi.org/10.1016/j.nlm.2015.12.010
Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S (2012) Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484(7394):381–385. https://doi.org/10.1038/nature11028
Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, Hen R (2014) Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83(1):189–201. https://doi.org/10.1016/j.neuron.2014.05.018
Lee JL, Hynds RE (2013) Divergent cellular pathways of hippocampal memory consolidation and reconsolidation. Hippocampus 23(3):233–244. https://doi.org/10.1002/hipo.22083
Campeau S, Dolan D, Watson SJ (2002) c-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress 5(2):121–130. https://doi.org/10.1080/10253890290027895
Kimura A, Donishi T, Sakoda T, Hazama M, Tamai Y (2003) Auditory thalamic nuclei projections to the temporal cortex in the rat. Neuroscience 117:1003–1016. https://doi.org/10.1016/s0306-4522(02)00949-1
Svobodová Burianová J, Syka J (2020) Postnatal exposure to an acoustically enriched environment alters the morphology of neurons in the adult rat auditory system. Brain Struct Funct 225:1979–1995. https://doi.org/10.1007/s00429-020-02104-8
Yuan K, Fink KL, Winer JA, Schreiner CE (2011) Local connection patterns of parvalbumin-positive inhibitory interneurons in rat primary auditory cortex. Hear Res 274:121–128. https://doi.org/10.1016/j.heares.2010.06.014
Silbersweig D, Stern E (1996) Functional neuroimaging of hallucinations in schizophrenia: toward an integration of bottom-up and top-down approaches. Mol Psychiatry 1:367–375
Gilbert CD, Sigman M (2007) Brain states: top-down influences in sensory processing. Neuron 54:677–696. https://doi.org/10.1016/j.neuron.2007.05.019
Sterzer P, Voss M, Schlagenhauf F, Heinz A (2019) Decision-making in schizophrenia: a predictive-coding perspective. Neuroimage 190:133–143. https://doi.org/10.1016/j.neuroimage.2018.05.074
Ramaker RC, Bowling KM, Lasseigne BN, Hagenauer MH, Hardigan AA, Davis NS, Gertz J, Cartagena PM, Walsh DM, Vawter MP, Jones EG, Schatzberg AF, Barchas JD, Watson SJ, Bunney BG, Akil H, Bunney WE, Li JZ, Cooper SJ, Myers RM (2017) Post-mortem molecular profiling of three psychiatric disorders. Genome Med 9(1):72. https://doi.org/10.1186/s13073-017-0458-5
Ananth J, Burgoyne KS, Gadasalli R, Aquino S (2001) How do the atypical antipsychotics work? J Psychiatry Neurosci 26:385–394
de Bartolomeis A, Buonaguro EF, Latte G, Rossi R, Marmo F, Iasevoli F, Tomasetti C (2017) Immediate-early genes modulation by antipsychotics: translational implications for a putative gateway to drug-induced long-term brain changes. Front Behav Neurosci 11:240. https://doi.org/10.3389/fnbeh.2017.00240
MacGibbon GA, Lawlor PA, Bravo R, Dragunow M (1994) Clozapine and haloperidol produce a differential pattern of immediate early gene expression in rat caudate-putamen, nucleus accumbens, lateral septum and islands of Calleja. Brain Res Mol Brain Res 23:21–32. https://doi.org/10.1016/0169-328x(94)90207-0
Robertson GS, Fibiger HC (1996) Effects of olanzapine on regional c-fos expression in rat forebrain. Neuropsychopharmacology 14:105–110. https://doi.org/10.1016/0893-133x(95)00196-k
Ohashi K, Hamamura T, Lee Y, Fujiwara Y, Suzuki H, Kuroda S (2000) Clozapine- and olanzapine-induced Fos expression in the rat medial prefrontal cortex is mediated by beta-adrenoceptors. Neuropsychopharmacology 23:162–169. https://doi.org/10.1016/s0893-133x(00)00105-6
Verma V, Rasmussen K, Dawe GS (2006) Effects of short-term and chronic olanzapine treatment on immediate early gene protein and tyrosine hydroxylase immunoreactivity in the rat locus coeruleus and medial prefrontal cortex. Neuroscience 143:573–585. https://doi.org/10.1016/j.neuroscience.2006.08.010
Liu S, Zhang F, Shugart YY, Yang L, Li X, Liu Z, Sun N, Yang C, Guo X, Shi J, Wang L, Cheng L, Zhang K, Yang T, Xu Y (2017) The early growth response protein 1-miR-30a-5p-neurogenic differentiation factor 1 axis as a novel biomarker for schizophrenia diagnosis and treatment monitoring. Transl Psychiatry 7:e998. https://doi.org/10.1038/tp.2016.268
Yamada K, Gerber DJ, Iwayama Y, Ohnishi T, Ohba H, Toyota T, Aruga J, Minabe Y, Tonegawa S, Yoshikawa T (2007) Genetic analysis of the calcineurin pathway identifies members of the EGR gene family, specifically EGR3, as potential susceptibility candidates in schizophrenia. Proc Natl Acad Sci USA 104:2815–2820. https://doi.org/10.1073/pnas.0610765104
Kyosseva SV (2004) Differential expression of mitogen-activated protein kinases and immediate early genes fos and jun in thalamus in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 28:997–1006. https://doi.org/10.1016/j.pnpbp.2004.05.017
Millard SJ, Bearden CE, Karlsgodt KH, Sharpe MJ (2021) The prediction-error hypothesis of schizophrenia: new data point to circuit-specific changes in dopamine activity. Neuropsychopharmacology. https://doi.org/10.1038/41386-021-01188-y
Hoffman AN, Parga A, Paode PR, Watterson LR, Nikulina EM, Hammer RP Jr, Conrad CD (2015) Chronic stress enhanced fear memories are associated with increased amygdala zif268 mRNA expression and are resistant to reconsolidation. Neurobiol Learn Mem 120:61–68. https://doi.org/10.1016/j.nlm.2015.02.004
Yu YH, Ou CY, Shyu BC, Huang ACW (2020) Basolateral amygdala but not medial prefrontal cortex contributes to chronic fluoxetine treatments for PTSD symptoms in mice. Behav Neurol 2020:8875087. https://doi.org/10.1155/2020/8875087
Covington HE III, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouson E, Ghose S, Taming CA, Neve RL, Deisseroth K, Nestler EJ (2010) Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci 30:16082–16090. https://doi.org/10.1523/JNEUROSCI.1731-10.2010
Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA (2012) A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord 140:113–124. https://doi.org/10.1016/j.jad.2011.10.023
Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W (1999) Activation of Heschl’s gyrus during auditory hallucinations. Neuron 22(3):615–621. https://doi.org/10.1016/s0896-6273(00)80715-1
Shergill SS, Brammer MJ, Williams SC, McGuire PK (2000) Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 57(11):1033–1038. https://doi.org/10.1001/archpsyc.57.11.1033
van de Ven VG, Formisano E, Röder CH, Prvulovic D, Bittner RA, Dietz DH, Dierks T, Federspiel A (2005) The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. Neuroimage 27(3):644–655. https://doi.org/10.1016/j.neuroimage.2005.04.041
Northoff G (2014) Are auditory hallucinations related to the brain’s resting state activity? A ‘Neurophenomenal Resting State Hypothesis.’ Clin Psychopharmacol Neurosci 12(3):189–195. https://doi.org/10.9758/cpn.2014.12.3.189
Kompus K, Westerhausen R, Hugdahl K (2011) The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia 49(12):3361–3369. https://doi.org/10.1016/j.neuropsychologia.2011.08.010
Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW (2007) Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry 64:521–529. https://doi.org/10.1001/archpsyc.64.5.521
Koshiyama D, Miyakoshi M, Joshi YB, Molina JL, Tanaka-Koshiyama K, Sprock J, Braff DL, Swerdlow NR, Light GA (2020) A distributed frontotemporal network underlies gamma-band synchronization impairments in schizophrenia patients. Neuropsychopharmacology 45(13):2198–2206. https://doi.org/10.1038/s41386-020-00806-5
Grent-’t-Jong T, Gajwani R, Gross J, Gumley AI, Krishnadas R, Lawrie SM, Schwannauer M, Schultze-Lutter F, Uhlhaas PJ (2021) 40-Hz auditory steady-state responses characterize circuit dysfunctions and predict clinical outcomes in clinical high-risk for psychosis participants: a magnetoencephalography study. Biol Psychiatry 90(6):419–429. https://doi.org/10.1016/j.biopsych.2021.03.018
Teale P, Carlson J, Rojas D, Reite M (2003) Reduced laterality of the source locations for generators of the auditory steady-state field in schizophrenia. Biol Psychiatry 54(11):1149–1153. https://doi.org/10.1016/s0006-3223(03)00411-6
Curcic-Blake B, Liemburg E, Vercammen A, Swart M, Knegtering H, Bruggeman R, Aleman A (2013) When Broca goes uninformed: reduced information flow to Broca’s area in schizophrenia patients with auditory hallucinations. Schizophr Bull 39(5):1087–1095. https://doi.org/10.1093/schbul/sbs107
Kompus K, Falkenberg LE, Bless JJ, Johnsen E, Kroken RA, Kråkvik B, Larøi F, Løberg EM, Vedul-Kjelsås E, Westerhausen R, Hugdahl K (2013) The role of the primary auditory cortex in the neural mechanism of auditory verbal hallucinations. Front Hum Neurosci 24(7):144. https://doi.org/10.3389/fnhum.2013.00144
Mørch-Johnsen L, Nesvåg R, Jørgensen KN, Lange EH, Hartberg CB, Haukvik UK, Kompus K, Westerhausen R, Osnes K, Andreassen OA, Melle I, Hugdahl K, Agartz I (2017) Auditory cortex characteristics in schizophrenia: associations with auditory hallucinations. Schizophr Bull 1:75–83. https://doi.org/10.1093/schbul/sbw130
Parker EM, Sweet RA (2018) Stereological assessments of neuronal pathology in auditory cortex in schizophrenia. Front Neuroanat 11:131. https://doi.org/10.3389/fnana.2017.00131
Acknowledgements
The authors would like to thank Dr. Shunsuke Hasegawa for technical assistance with the experiments. This research was supported by Grant-in-Aid for Scientific Research on Innovative Areas (18H05429, H.N.) and for Challenging Research (21K18242, H.N.), the Japan Agency for Medical Research and Development (AMED, JP21wm0425019, A.K.), the Strategic Research Program for Brain Sciences from AMED (JP21wm0425019, H.Y. and JP21dm0207074, Y.K.), the Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (JP21H00180, Y.K.), the Grants-in-Aid for Scientific Research (C) (19K08053, Y.K.), the Ministry of Education, Culture, Sports, Science, and Technology Supported Program for the Strategic Research Foundation at Private Universities (S1311017, RK-M), and the Collaborative Research Project of Brain Research Institute, Niigata University (201917, Y.K.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. YI, SK, TS and HN contributed to the study conception and design. YI, RK-M, and HS performed experiments. Materials were prepared or collected by RG, HT, YK, MH, AN, RI, RS, HY, AK. The first draft of the manuscript was written by YI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iwakura, Y., Kawahara-Miki, R., Kida, S. et al. Elevation of EGR1/zif268, a Neural Activity Marker, in the Auditory Cortex of Patients with Schizophrenia and its Animal Model. Neurochem Res 47, 2715–2727 (2022). https://doi.org/10.1007/s11064-022-03599-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03599-9