Abstract
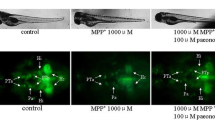
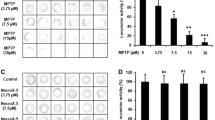
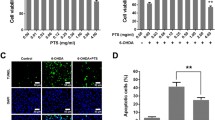
Parkinson’s disease (PD) is one of the most common forms of neurodegenerative diseases and research on potential therapeutic agents for PD continues. Rotenone is a neurotoxin that can pass the blood–brain barrier and is used to generate PD models in experimental animals. Boron is a microelement necessary for neural activity in the brain. Antioxidant, non-cytotoxic, anti-genotoxic, anti-carcinogenic effects of boric acid, the salt compound of boron has been reported before. Boronic acids have been approved for treatment by FDA and are included in drug discovery studies and pyridine boronic acids are a subclass of heterocyclic boronic acids used in drug design and discovery as substituted pyridines based on crystal engineering principles. The aim of our study was to determine the effect of 3-pyridinylboronic acid in rotenone-exposed zebrafish embryos, focusing on oxidant-antioxidant parameters and gene expression levels of nuclear factor erythroid 2-related factor 2 (Nrf2) target genes gclm, gclc, hmox1a, nqo1, and PD related genes, brain-derived neurotrophic factor, dj1, and tnfα. Zebrafish embryos were exposed to Rotenone (10 μg/l); Low Dose 3-Pyridinylboronic acid (100 μM); High Dose 3-Pyridinylboronic acid (200 μM); Rotenone + Low Dose-3-Pyridinylboronic acid (10 μg/l + 100 μM); Rotenone + High Dose-3-Pyridinylboronic acid (10 μg/l + 200 μM) in well plates for 96 h post-fertilization (hpf). Our study showed for the first time that 3-pyridinylboronic acid, as a novel sub-class of the heterocyclic boronic acid compound, improved locomotor activities, ameliorated oxidant-antioxidant status by decreasing LPO and NO levels, and normalized the expressions of bdnf, dj1, tnf⍺ and Nrf2 target genes hmox1a and nqo1 in rotenone exposed zebrafish embryos. On the other hand, it caused the deterioration of the oxidant-antioxidant balance in the control group through increased lipid peroxidation, nitric oxide levels, and decreased antioxidant enzymes. We believe that these results should be interpreted in the context of the dose-toxicity and benefit-harm relationship of the effects of 3-pyridinylboronic.






Similar content being viewed by others
Data Availability
Data will be available on reasonable request.
References
Üstündaǧ A, Behm C, Föllmann W, Duydu Y, Degen GH (2014) Protective effect of boric acid on lead- and cadmium-induced genotoxicity in V79 cells. Arch Toxicol 88:1281–1289. https://doi.org/10.1007/s00204-014-1235-5
Herrero M, Ibáñez E, Cifuentes A (2005) Analysis of natural antioxidants by capillary electromigration methods. J Sep Sci 28:883–897. https://doi.org/10.1002/jssc.200400104
Türkez H, Geyikoğlu F, Tatar A, Keleş S, Özkan A (2007) Effects of some boron compounds on peripheral human blood. Z Naturforsch C J Biosci 62:889–896. https://doi.org/10.1016/j.etp.2010.06.011
Turkez H (2008) Effects of boric acid and borax on titanium dioxide genotoxicity. J Appl Toxicol 28:658–664. https://doi.org/10.1002/jat.1318
Turkez H, Geyikoglu F (2010) Boric acid: a potential chemoprotective agent against aflatoxin b1toxicity in human blood. Cytotechnology 62:157–165
Penland JG (1998) The importance of boron nutrition for brain and psychological function. Biol Trace Elem Res 66:299–317. https://doi.org/10.1007/bf02783144
Białek M, Czauderna M, Krajewska KA, Przybylski W (2019) Selected physiological effects of boron compounds for animals and humans: a review. J Anim Feed Sci 28:307–320. https://doi.org/10.22358/jafs/114546/2019
Plescia J, Moitessier N (2020) Design and discovery of boronic acid drugs. Eur J Med Chem 195:112270. https://doi.org/10.1016/j.ejmech.2020.112270
Liu Y, Milo LJ Jr, Lai JH (2013) Recent progress in the synthesis of pyridinylboronic acids and esters. ARKIVOC 1:135–153. https://doi.org/10.3998/ark.5550190.0014.104
Fontaine F, Héquet A, Voisin-Chiret AS, Bouillon A, Lesnard A, Cresteil T, Jolivalt C, Rault S (2015) Boronic species as promising inhibitors of the Staphylococcus aureus NorA efflux pump: study of 6-substituted pyridine-3-boronic acid derivatives. Eur J Med Chem 95:185–198. https://doi.org/10.1016/j.ejmech.2015.02.056
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 3(4):461–491. https://doi.org/10.3233/JPD-130230
Cuadrado A, Moreno-Murciano P, Pedraza-Chaverri J (2009) The transcription factor Nrf2 as a new therapeutic target in Parkinson’s disease. Expert Opin Ther Targets 13(3):319–329
Vaz RL, Outeiro TF, Ferreira JJ (2018) Zebrafish as an animal model for drug discovery in Parkinson’s disease and other movement disorders: a systematic review. Front Neurol 9:347. https://doi.org/10.3389/fneur.2018.00347
Ünal İ, Emekli-Alturfan E (2019) Fishing for Parkinson’s disease: a review of the literature. J Clin Neurosci 62:1–6. https://doi.org/10.1016/j.jocn.2019.01.015
Bhurtel S, Katila N, Srivastav S, Neupane S, Choi DY (2019) Mechanistic comparison between MPTP and rotenone neurotoxicity in mice. Neurotoxicology 71:113–121. https://doi.org/10.1016/j.neuro.2018.12.009
Giordano S, Lee J, Darley-Usmar VM, Zhang J (2012) Distinct effects of rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on cellular bioenergetics and cell death. PLoS ONE 7(9):e44610. https://doi.org/10.1371/journal.pone.0044610
Nicklas WJ, Vyas I, Heikkila RE (1985) Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci 36:2503–2508. https://doi.org/10.1016/0024-3205(85)90146-8
Üstündağ FD, Ünal İ, Cansız D, Üstündağ ÜV, Subaşat HK, Alturfan AA, Tiber PM, Emekli-Alturfan E (2020) 3-Pyridinylboronic acid normalizes the effects of 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure in zebrafish embryos. Drug Chem Toxicol. https://doi.org/10.1080/01480545.2020.1795189
Yahsi Y, Gungor E, Kara H (2015) Chlorometallate-pyridinium boronic acid salts for Crystal engineering: synthesis of one-, two-, and three-dimensional hydrogen bond networks. Cryst Growth Des 15:2652–2660. https://doi.org/10.1021/cg501769b
Westerfield M (1995) The zebrafish book: a guide for the laboratory use of zebrafish. University of Oregon Press, Eugene, OR
Goody MF, Kelly MW, Reynolds CJ, Khalil A, Crawford BD, Henry CA (2012) NAD + biosynthesis ameliorates a zebrafish model of muscular dystrophy. PLoS Biol 10:e1001409. https://doi.org/10.1371/journal.pbio.1001409
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real- time quantitative PCR and the 2ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Yagi K (1984) Assay for blood plasma or serum. Methods Enzymol 105:328–337. https://doi.org/10.1016/S0076-6879(84)05042-4
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide—Biol Chem 5:62–71. https://doi.org/10.1006/niox.2000.0319
Beutler E (1975) Glutathione in red cell metabolism : a manual of Biochemical methods, 2nd edn. Grune and Stratton, NY, pp 112–114
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405. https://doi.org/10.1016/S0076-6879(81)77053-8
Vomund S, Schäfer A, Parnham MJ, Brüne B, von Knethen A (2017) Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci 18:2772. https://doi.org/10.3390/ijms18122772
Ramsay RR, Singer TP (1986) Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J Biol Chem 261:7585–7587. https://doi.org/10.1016/S0021-9258(19)57434-8
Moscovitz O, Ben-Nissan G, Fainer I, Pollack D, Mizrachi L, Sharon M (2015) The Parkinson’s-associated protein DJ-1 regulates the 20S proteasome. Nat Commun 6:6609. https://doi.org/10.1038/ncomms7609
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
Dhakshinamoorthy S, Long DJ, Jaiswal AK (2000) Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr Top Cell Regul. 36:201–216
Kurutas EB (2016) The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 15(1):71. https://doi.org/10.1186/s12937-016-0186-5
Oh ET, Park HJ (2015) Implications of NQO1 in cancer therapy. BMB Rep 48(11):609–617
Poss KD, Tonegawa S (1997) Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA 94(20):10925–10930
Krejsa CM, Franklin CC, White CC, Ledbetter JA, Schieven GL, Kavanagh TJ (2010) Rapid activation of glutamate cysteine ligase following oxidative stress. J Biol Chem 285(21):16116–16124
Fujii J, Ito JI, Zhang X, Kurahashi T (2011) Unveiling the roles of the glutathione redox system in vivo by analyzing genetically modified mice. J Clin Biochem Nutr 49(2):70–78. https://doi.org/10.3164/jcbn.10-138SR
Hunt CD, Idso JP (1999) Dietary boron as a phys-iological regulator of the normal inflammatory response: a review and current research progress. J Trace Elem Exp Med 12:221–233. https://doi.org/10.1002/(SICI)1520-670X(1999)12:3%3C221::AIDJTRA6%3E3.0.CO;2-X
Kucukkurt I, Ince S, Demirel HH, Turkmen R, Akbel E, Celik Y (2015) The effects of boron on arsenic-induced lipid peroxidation and antioxidant status in male and female rats. J Biochem Mol Toxicol 29(12):564–571
Nielsen FH (2009) Boron deprivation decreases liver S-adenosylmethionine and spermidine and increases plasma homocysteine and cysteine in rats. J Trace Elem Med Biol 23(3):204–213
Hoffman DJ, Sanderson CJ, Le Captain LJ, Crom-artie E, Pendleton GW (1992) Interactive ef-fects of selenium, methionine, and dietary protein onsurvival, growth, and physiology in mallard ducklings. Arch Environ Contam Toxicol 23:163–171. https://doi.org/10.1007/BF00213302
Al-Saleh IA, Al-Doush I (1997) Selenium levelsin wheat grains grown in Saudi Arabia. Bull Environ Contam Toxicol 59:590–594
Gülsoy N, Yavas C, Mutlu Ö (2015) Genotoxic effects of boric acid and borax in zebrafish, Danio rerio using alkaline comet assay. EXCLI J. 14:890–899. https://doi.org/10.17179/excli2015-404
Heindel JJ, Price CJ, Schwetz BA (1994) The developmental toxicity of boric acid in mice, rats, and rabbits. Environmental Health Perspectives 102(Suppl 7):107–112
Naghii MR, Mofid M, Asgari AR, Hedayati M, Daneshpour MS (2011) Comparative effects of daily and weekly boron supplementation on plasma steroid hormones and proinflammatory cytokines. J Trace Elem Med Biol 25:54–58. https://doi.org/10.1016/j.jtemb.2010.10.001
Stefanis L, Burke RE, Greene LA (1997) Apoptosis in neurodegenerative disorders. Curr Opin Neurol 10:299–305
Penland JG (1994) Dietary boron, brain function, and cognitive performance. Environ Health Perspect 102:65–72. https://doi.org/10.1289/ehp.94102s765
Pizzorno L (2015) Nothing boring about boron. Integr Med (Encinitas) 14:35–48
Jiang L, Zhang H, Wang C, Ming F, Shi X, Yang M (2019) Serum level of brain-derived neurotrophic factor in Parkinson’s disease: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 10(88):168–174. https://doi.org/10.1016/j.pnpbp.2018.07.010
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no conflicts of interest.
Ethical Approval
As the zebrafish embryos used were no older than 5 days old, no ethical approval was required for the protocols applied as stated by the Council of Europe (1986), Directive 86/609/EEC.
Consent to Participate
All the authors have agreed for authorship, read and approved the manuscript, and given consent to participate.
Consent for Publication
All the authors have agreed for authorship, read and approved the manuscript, and given consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Üstündağ, F.D., Ünal, İ., Üstündağ, Ü.V. et al. 3-Pyridinylboronic Acid Ameliorates Rotenone-Induced Oxidative Stress Through Nrf2 Target Genes in Zebrafish Embryos. Neurochem Res 47, 1553–1564 (2022). https://doi.org/10.1007/s11064-022-03548-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03548-6