Abstract
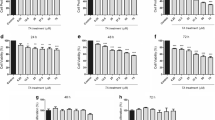
Glioblastoma (GB) is a highly aggressive and invasive brain tumor; its treatment remains palliative. Tannic acid (TA) is a polyphenol widely found in foods and possesses antitumor and neuroprotective activities. This study aimed to investigate the effect of TA on oxidative stress parameters and the activity of ectonucleotidases in the serum, platelets, and lymphocytes and/or in the brain of rats with preclinical GB. Rats with GB were treated intragastrically with TA (50 mg/kg/day) for 15 days or with a vehicle. In the platelets of the animals with glioma, the adenosine triphosphate (ATP) and adenosine monophosphate (AMP) hydrolysis and the catalase (CAT) activity decreased. Besides, the adenosine diphosphate (ADP) hydrolysis, adenosine (Ado) deamination, and the reactive oxygen species (ROS) and nitrite levels were increased in glioma animals; however, TA reversed ROS and nitrite levels and AMP hydrolysis alterations. In lymphocytes from animals with glioma, the ATP and ADP hydrolysis, as well as Ado deamination were increased; TA treatment countered this increase. In the brain of the animals with glioma, the ROS, nitrite, and thiobarbituric acid reactive substance (TBARS) levels increased and the thiol (SH) levels and CAT and superoxide dismutase (SOD) activities were decreased; TA treatment decreased the ROS and TBARS levels and restored the SOD activity. In the serum of the animals with glioma, the ATP hydrolysis decreased; TA treatment restored this parameter. Additionally, the ROS levels increased and the SH and SOD activity decreased by glioma implant; TA treatment enhanced nitrite levels and reversed SOD activity. Altogether, our results suggest that TA is an important target in the treatment of GB, as it modulates purinergic and redox systems.






Similar content being viewed by others
References
Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin 70(4):299–312. https://doi.org/10.3322/caac.21613
Pande A, Rajaraman N, Sadiya N, Patil S, Pandian S, Adhithyan R, Rajendran B, Jalali R, Ghosh S (2020) Spinal drop metastasis of glioblastoma-two case reports, clinicopathologic features, current modalities of evaluation, and treatment with a review of the literature. World Neurosurg 146:261–269. https://doi.org/10.1016/j.wneu.2020.10.023
Ou A, Yung WKA, Majd N (2020) Molecular mechanisms of treatment resistance in glioblastoma. Int J Mol Sci 22(1):351. https://doi.org/10.3390/ijms22010351
Olivier C, Oliver L, Lalier L, Vallette FM (2021) Drug resistance in glioblastoma: the two faces of oxidative stress. Front Mol Biosci 7:620677. https://doi.org/10.3389/fmolb.2020.620677
Pietrobono D, Giacomelli C, Marchetti L, Martini C, Trincavelli ML (2020) High adenosine extracellular levels induce glioblastoma aggressive traits modulating the mesenchymal stromal cell secretome. Int J Mol Sci 21(20):7706. https://doi.org/10.3390/ijms21207706
Azambuja JH, Gelsleichter NE, Beckenkamp LR, Iser IC, Fernandes MC, Figueiró F, Battastini AMO, Scholl JN, de Oliveira FH, Spanevello RM, Sévigny J, Wink MR, Stefani MA, Teixeira HF, Braganhol E (2019) CD73 downregulation decreases in vitro and in vivo glioblastoma growth. Mol Neurobiol 56(5):3260–3279. https://doi.org/10.1007/s12035-018-1240-4
Zhou Y, Wang L, Wang C, Wu Y, Chen D, Lee TH (2020) Potential implications of hydrogen peroxide in the pathogenesis and therapeutic strategies of gliomas. Arch Pharm Res 43(2):187–203. https://doi.org/10.1007/s12272-020-01205-6
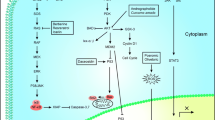
Idzko M, Ferrari D, Eltzsching HK (2014) Nucleotide signaling during inflammation. Nature 15:310–317. https://doi.org/10.1038/nature13085
Di Virgilio F, Adinolfi E (2017) Extracellular purines, purinergic receptors and tumor growth. Oncogene 36:293–303. https://doi.org/10.1038/onc.2016.206
Zimmermann H, Zebisch M, Sträter N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8(1):105–106. https://doi.org/10.1007/s11302-011-9256-5
Linhares P, Carvalho B, Vaz R, Costa BM (2020) Glioblastoma: is there any blood biomarker with true clinical relevance? Int J Mol Sci 21(16):5809. https://doi.org/10.3390/ijms21165809
Muir M, Gopakumar S, Traylor J, Lee S, Rao G (2020) Glioblastoma multiforme: novel therapeutic targets. Expert Opin Ther Targets 24(7):605–614. https://doi.org/10.1080/14728222.2020.1762568
Deng LJ, Qi M, Li N, Lei YH, Zhang DM, Chen JX (2020) Natural products and their derivatives: promising modulators of tumor immunotherapy. J Leukoc Biol 108(2):493–508. https://doi.org/10.1002/JLB.3MR0320-444R
Talib WH, Alsalahat I, Daoud S, Abutayeh RF, Mahmod AI (2020) Plant-derived natural products in cancer research: extraction, mechanism of action, and drug formulation. Molecules 25(22):5319. https://doi.org/10.3390/molecules25225319
Li H, Krstin S, Wink M (2018) Modulation of multidrug resistant in cancer cells by EGCG, tannic acid and curcumin. Phytomedicine 50:213–222. https://doi.org/10.1016/j.phymed.2018.09.169
Bona NP, Pedra NS, Azambuja JH, Soares MSP, Spohr L, Gelsleichter NE, de Meine MB, Sekine FG, Mendonça LT, de Oliveira FH, Braganhol E, Spanevello RM, da Silveira EF, Stefanello FM (2020) Tannic acid elicits selective antitumoral activity in vitro and inhibits cancer cell growth in a preclinical model of glioblastoma multiforme. Metab Brain Dis 35(2):283–293. https://doi.org/10.1007/s11011-019-00519-9
Nagesh PKB, Chowdhury P, Hatami E, Jain S, Dan N, Kashyap VK, Chauhan SC, Jaggi M, Yallapu MM (2020) Tannic acid inhibits lipid metabolism and induce ROS in prostate cancer cells. Sci Rep 10(1):980. https://doi.org/10.1038/s41598-020-57932-9
Zhang J, Chen D, Han DM, Cheng YH, Dai C, Wu XJ, Che FY, Heng XY (2018) Tannic acid mediated induction of apoptosis in human glioma Hs 683 cells. Oncol Lett 15(5):6845–6850. https://doi.org/10.3892/ol.2018.8197
Gerzson MFB, Pacheco SM, Soares MSP, Bona NP, Oliveira PS, Azambuja JH, da Costa P, Gutierres JM, Carvalho FB, Morsch VM, Spanevello RM, Stefanello FM (2019) Effects of tannic acid in streptozotocin-induced sporadic Alzheimer’s disease: insights into memory, redox status, Na+, K+-ATPase and acetylcholinesterase activity. Arch Physiol Biochem. https://doi.org/10.1080/13813455.2019.1673430
Gerzson MFB, Bona NP, Soares MSP, Teixeira FC, Rahmeier FL, Carvalho FB, da Cruz FM, Onzi G, Lenz G, Gonçales RA, Spanevello RM, Stefanello FM (2020) Tannic acid ameliorates STZ-induced Alzheimer’s disease-like impairment of memory, neuroinflammation, neuronal death and modulates Akt expression. Neurotox Res 37:1009–1017. https://doi.org/10.1007/s12640-020-00167-3
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Böyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Investig 97:77–89
Jaques JA, Peres JRR, Ruchel JB, Gutierres J, Bairros AV, Gomes ILF, Almeida SCL, Mello CDB, Chitolina MRS, Morsch VM, Leal DB (2011) A method for isolation of rat lymphocyte-rich mononuclear cells from lung tissue useful for determination of nucleoside triphosphate diphosphohydrolase activity. Anal Biochem 410:34–39. https://doi.org/10.1016/j.ab.2010.10.039
Lunkes GIL, Lunkes DS, Morsch VM et al (2004) NTPDase and 5′- nucleotidase activities in rats with alloxan-induced diabetes. Diabetes Res Clin Pract 65:1–6. https://doi.org/10.1016/j.diabres.2003.11.016
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Fürstenau CR, Trentin DS, Gossenheimer AN et al (2008) Ectonucleotidase activities are altered in serum and platelets of L-NAME-treated rats. Blood Cells Mol Dis 41:223–229. https://doi.org/10.1016/j.bcmd.2008.04.009
Chan K, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca2+–ATPase activity. Analyt Biochem 157:375–378. https://doi.org/10.1016/0003-2697(86)90640-8
Leal DB, Streher CA, Neu TN, Bittencourt FP, Leal CA, da Silva JE, Morsch VM, Schetinger MR (2005) Characterization of NTPDase (NTPDase 1: ectoapyrase; ectodiphosphohydrolase; CD39; E.C. 3.6.1.5) activity in human lymphocytes. Biochim Biophys Acta 1721:9–11. https://doi.org/10.1016/j.bbagen.2004.09.006
Pilla C, Emanuelli T, Frassetto SS, Battastini AMO, Dias RD, Sarkis JJF (1996) ATP diphosphohydrolase activity (apyrase E.C. 3.6.1.5) in human blood platelets. Platelets 7:225–230. https://doi.org/10.3109/09537109609023582
Giusti G, Galanti B (1984) Colorimetric method. Adenosine deaminase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 3rd edn. Wiley, Weinheim, pp 315–3233
Ali SF, LeBel CP, Bondy SC (1992) Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 13:637–648
Stuehr DJ, Nathan CF (1989) Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med 169:1543–1555. https://doi.org/10.1084/jem.169.5.1543
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145. https://doi.org/10.1016/S0304-3940(01)01636-6
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. https://doi.org/10.1016/0076-6879(90)86134-H
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Marx S, Splittstöhser M, Kinnen F, Moritz E, Joseph C, Paul S, Paland H, Seifert C, Marx M, Böhm A, Schwedhelm E, Holzer K, Singer S, Ritter CA, Bien-Möller S, Schroeder HWS, Rauch BH (2018) Platelet activation parameters and platelet-leucocyte-conjugate formation in glioblastoma multiforme patients. Oncotarget 9(40):25860–25876. https://doi.org/10.18632/oncotarget.25395
Yersal Ö, Odabaşi E, Özdemir Ö, Kemal Y (2018) Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with glioblastoma. Mol Clin Oncol 9(4):453–458. https://doi.org/10.3892/mco.2018.1695
Sol N, In’t Veld SGJG, Vancura A, Tjerkstra M, Leurs C, Rustenburg F, Schellen P, Verschueren H, Post E, Zwaan K, Ramaker J, Wedekind LE, Tannous J, Ylstra B, Killestein J, Mateen F, Idema S, de Witt Hamer PC, Navis AC, Leenders WPJ, Hoeben A, Moraal B, Noske DP, Vandertop WP, Nilsson RJA, Tannous BA, Wesseling P, Reijneveld JC, Best MG, Wurdinger T (2020) Tumor-educated platelet RNA for the detection and (pseudo)progression monitoring of glioblastoma. Cell Rep Med 1(7):100101. https://doi.org/10.1016/j.xcrm.2020.100101
Gachet C, Hechler B (2020) Platelet purinergic receptors in thrombosis and inflammation. Hamostaseologie. https://doi.org/10.1055/a-1113-0711
Atkinson B, Dwyer K, Enjyoji K, Robson SC (2006) Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: potential as therapeutic targets. Blood Cells Mol Dis 36(2):217–222. https://doi.org/10.1016/j.bcmd.2005.12.025
Marx S, Xiao Y, Baschin M, Splittstöhser M, Altmann R, Moritz E, Jedlitschky G, Bien-Möller S, Schroeder HWS, Rauch BH (2019) The role of platelets in cancer pathophysiology: focus on malignant glioma. Cancers (Basel) 11(4):569. https://doi.org/10.3390/cancers11040569
Campanella R, Guarnaccia L, Cordiglieri C, Trombetta E, Caroli M, Carrabba G, La Verde N, Rampini P, Gaudino C, Costa A, Luzzi S, Mantovani G, Locatelli M, Riboni L, Navone SE, Marfia G (2020) Tumor-educated platelets and angiogenesis in glioblastoma: another brick in the wall for novel prognostic and targetable biomarkers, changing the vision from a localized tumor to a systemic pathology. Cells 9(2):294. https://doi.org/10.3390/cells9020294
Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wöhrer A, Dieckmann K, Filipits M, Brandstetter A, Weller M, Kurscheid S, Hegi ME, Zielinski CC, Marosi C, Hainfellner JA, Preusser M, Wick W (2015) Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol 17(8):1064–1075. https://doi.org/10.1093/neuonc/nou307
Stagg J, Smyth MJ (2010) Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 29(39):5346–5358. https://doi.org/10.1038/onc.2010.292onc2010292
Drill M, Powell KL, Kan LK, Jones NC, O’Brien TJ, Hamilton JA, Monif M (2020) Inhibition of purinergic P2X receptor 7 (P2X7R) decreases granulocyte-macrophage colony-stimulating factor (GM-CSF) expression in U251 glioblastoma cells. Sci Rep 10(1):14844. https://doi.org/10.1038/s41598-020-71887-x
Ledur PF, Villodre ES, Paulus R, Cruz LA, Flores DG, Lenz G (2012) Extracellular ATP reduces tumor sphere growth and cancer stem cell population in glioblastoma cells. Purinergic Signal 8(1):39–48. https://doi.org/10.1007/s11302-011-9252-9
Nie F, Liang Y, Jiang B, Li X, Xun H, He W, Lau HT, Ma X (2016) Apoptotic effect of tannic acid on fatty acid synthase over-expressed human breast cancer cells. Tumour Biol 37(2):2137–2143. https://doi.org/10.1007/s13277-015-4020-z
Nagesh PKB, Hatami E, Chowdhury P, Kashyap VK, Khan S, Hafeez BB, Chauhan SC, Jaggi M, Yallapu MM (2018) Tannic acid induces endoplasmic reticulum stress-mediated apoptosis in prostate cancer. Cancers (Basel) 10(3):68. https://doi.org/10.3390/cancers10030068
Mhlanga P, Perumal PO, Somboro AM, Amoako DG, Khumalo HM, Khan RB (2019) Mechanistic insights into oxidative stress and apoptosis mediated by tannic acid in human liver hepatocellular carcinoma cells. Int J Mol Sci 20(24):6145. https://doi.org/10.3390/ijms20246145
Sp N, Kang DY, Jo ES, Rugamba A, Kim WS, Park YM, Hwang DY, Yoo JS, Liu Q, Jang KJ, Yang YM (2020) Tannic acid promotes TRAIL-induced extrinsic apoptosis by regulating mitochondrial ROS in human embryonic carcinoma cells. Cells 9(2):282. https://doi.org/10.3390/cells9020282
Vavaev AV, Buryachkovskaya LI, Uchitel IA, Tishchenko EG, Maksimenko AV (2012) Effect of hydrogen peroxide and catalase derivatives on functional activity of platelets. Bull Exp Biol Med 152:307–312. https://doi.org/10.1007/s10517-012-1515-0
Kim DA, Choi HS, Ryu ES, Ko J, Shin HS, Lee JM, Chung H, Jun E, Oh ES, Kang DH (2019) Tannic acid attenuates the formation of cancer stem cells by inhibiting NF-κB-mediated phenotype transition of breast cancer cells. Am J Cancer Res 9(8):1664–1681
Darvin P, Joung YH, Kang DY, Sp N, Byun HJ, Hwang TS, Sasidharakurup H, Lee CH, Cho KH, Park KD, Lee HK, Yang YM (2017) Tannic acid inhibits EGFR/STAT1/3 and enhances p38/STAT1 signalling axis in breast cancer cells. J Cell Mol Med 21(4):720–734. https://doi.org/10.1111/jcmm.13015
Acknowledgements
This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico and Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES) – Finance code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bona, N.P., Soares, M.S.P., Pedra, N.S. et al. Tannic Acid Attenuates Peripheral and Brain Changes in a Preclinical Rat Model of Glioblastoma by Modulating Oxidative Stress and Purinergic Signaling. Neurochem Res 47, 1541–1552 (2022). https://doi.org/10.1007/s11064-022-03547-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03547-7