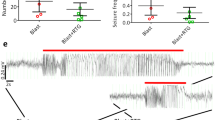
Abstract
Post-traumatic epilepsy (PTE) is a serious complication that can occur following traumatic brain injury (TBI). Sustained secondary changes after TBI promote the process of PTE. Here, we aim to evaluate changes in behavior, electrocorticogram, and histomorphology in rats following chronic TBI models. We observed intensive 7–8 Hz spike-wave-discharges (SWDs) at frontal recording sites and quantified them in SD rats with different degrees of TBI and compared them with age-matched sham rats to evaluate the association between SWDs and injury severity. Notably, although SWDs were even presented in the sham group, the number and duration of events were much lower than those in the TBI groups. SWDs have numerous similarities to absence seizures, such as abrupt onset, termination, and lack of postictal suppression, which may be the nonconvulsive characteristics of PTE. Retigabine, a novel antiepileptic drug, is ineffective in reducing SWDs. In addition, we examined chronic histopathological changes in TBI rats. Rats subjected to moderate and severe TBI exhibited significantly impaired neurological function, which was accompanied by marked cortical injury, hippocampus deformation, reactive gliosis, and mossy fiber sprouting. Long-term progressive structural changes in the brain are one of the characteristics of epileptogenesis after TBI. Our study provided the potential value of epileptiform SWDs in reflecting the nonconvulsive characteristic of PTE and highlighted the vital role of chronic pathological changes, such as reactive gliosis, in promoting the epileptogenesis following TBI.








Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TBI:
-
Traumatic brain injury
- PTE:
-
Post-traumatic epilepsy
- CCI:
-
Controlled cortical impact
- DG:
-
Dentate gyrus
- ASs:
-
Absence seizures
- SWDs:
-
Spike-wave-discharges
- ECoG:
-
Electrocorticogram
- GAERS:
-
Genetic Absence Epilepsy Rats from Strasbourg
- WAG/Rij:
-
Wistar Albino Glaxo Rats from Rijswijk
- AP:
-
Anteroposterior
- ML:
-
Mediolateral
- mNSS:
-
Modified Neurological Severity Score
- PFA:
-
Paraformaldehyde
- Iba1:
-
Ionized calcium binding adaptor molecule 1
- GFAP:
-
Glial fibrillary acidic protein
- MFS:
-
Mossy fiber sprouting
- ZnT3:
-
Zinc transporter 3
- FPI:
-
Fluid percussion injury
- GABA:
-
Gamma-aminobutyric acid
- eGABAAR:
-
Extrasynaptic GABAA receptors
References
Lucke-Wold BP, Nguyen L, Turner RC, Logsdon AF, Chen YW, Smith KE, Huber JD, Matsumoto R, Rosen CL, Tucker ES, Richter E (2015) Traumatic brain injury and epilepsy: underlying mechanisms leading to seizure. Seizure 33:13–23. https://doi.org/10.1016/j.seizure.2015.10.002
Bolkvadze T, Pitkanen A (2012) Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J Neurotrauma 29:789–812. https://doi.org/10.1089/neu.2011.1954
Hunt RF, Scheff SW, Smith BN (2009) Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp Neurol 215:243–252. https://doi.org/10.1016/j.expneurol.2008.10.005
Statler KD, Scheerlinck P, Pouliot W, Hamilton M, White HS, Dudek FE (2009) A potential model of pediatric posttraumatic epilepsy. Epilepsy Res 86:221–223. https://doi.org/10.1016/j.eplepsyres.2009.05.006
Allan J, Hartman PG, Crane-Robinson C, Aviles FX (1980) The structure of histone H1 and its location in chromatin. Nature 288:675–679
Kelly KM, Miller ER, Lepsveridze E, Kharlamov EA, McHedlishvili Z (2015) Posttraumatic seizures and epilepsy in adult rats after controlled cortical impact. Epilepsy Res 117:104–116. https://doi.org/10.1016/j.eplepsyres.2015.09.009
Pitkänen A, McIntosh TK (2006) Animal models of post-traumatic epilepsy. J Neurotrauma 23:241–261. https://doi.org/10.1089/neu.2006.23.241
Crunelli V, Leresche N (2002) Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci 3:371–382. https://doi.org/10.1038/nrn811
Blumenfeld H (2005) Cellular and network mechanisms of spike-wave seizures. Epilepsia 46(Suppl 9):21–33. https://doi.org/10.1111/j.1528-1167.2005.00311.x
Coenen AM, Drinkenburg WH, Inoue M, van Luijtelaar EL (1992) Genetic models of absence epilepsy, with emphasis on the WAG/Rij strain of rats. Epilepsy Res 12:75–86. https://doi.org/10.1016/0920-1211(92)90029-s
Kharlamov EA, Jukkola PI, Schmitt KL, Kelly KM (2003) Electrobehavioral characteristics of epileptic rats following photothrombotic brain infarction. Epilepsy Res 56:185–203. https://doi.org/10.1016/j.eplepsyres.2003.09.005
Kelly KM, Kharlamov A, Hentosz TM, Kharlamova EA, Williamson JM, Bertram EH 3rd, Kapur J, Armstrong DM (2001) Photothrombotic brain infarction results in seizure activity in aging Fischer 344 and Sprague Dawley rats. Epilepsy Res 47:189–203. https://doi.org/10.1016/s0920-1211(01)00294-7
Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M (2001) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32:2682–2688. https://doi.org/10.1161/hs1101.098367
Rodgers KM, Dudek FE, Barth DS (2015) Progressive, seizure-like, spike-wave discharges are common in both injured and uninjured Sprague-Dawley rats: implications for the fluid percussion injury model of post-traumatic epilepsy. J Neurosci 35:9194–9204. https://doi.org/10.1523/jneurosci.0919-15.2015
Komoltsev IG, Frankevich SO, Shirobokova NI, Volkova AA, Levshina IP, Novikova MR, Manolova AO, Gulyaeva NV (2021) Differential early effects of traumatic brain injury on spike-wave discharges in Sprague-Dawley rats. Neurosci Res 166:42–54. https://doi.org/10.1016/j.neures.2020.05.005
Taylor JA, Reuter JD (2019) Spontaneous recurrent absence seizure-like events in wild-caught rats. J Neurosci 39:4829–4841. https://doi.org/10.1523/jneurosci.1167-18.2019
Large CH, Sokal DM, Nehlig A, Gunthorpe MJ, Sankar R, Crean CS, Vanlandingham KE, White HS (2012) The spectrum of anticonvulsant efficacy of retigabine (ezogabine) in animal models: implications for clinical use. Epilepsia 53:425–436. https://doi.org/10.1111/j.1528-1167.2011.03364.x
Mazarati A, Wu J, Shin D, Kwon YS, Sankar R (2008) Antiepileptogenic and antiictogenic effects of retigabine under conditions of rapid kindling: an ontogenic study. Epilepsia 49:1777–1786. https://doi.org/10.1111/j.1528-1167.2008.01674.x
Buckmaster PS, Lew FH (2011) Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci 31:2337–2347. https://doi.org/10.1523/jneurosci.4852-10.2011
Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD (1997) Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci USA 94:12676–12681. https://doi.org/10.1073/pnas.94.23.12676
Campbell JN, Gandhi A, Singh B, Churn SB (2014) Traumatic brain injury causes a tacrolimus-sensitive increase in non-convulsive seizures in a rat model of post-traumatic epilepsy. Int. J. Neurol. Brain Disord. 2:1–11
D’Ambrosio R, Miller JW (2010) What is an epileptic seizure? Unifying definitions in clinical practice and animal research to develop novel treatments. Epilepsy currents 10:61–66. https://doi.org/10.1111/j.1535-7511.2010.01358.x
Tenney JR, Glauser TA (2013) The current state of absence epilepsy: can we have your attention? Epilepsy currents 13:135–140. https://doi.org/10.5698/1535-7511-13.3.135
Blumenfeld H (2005) Consciousness and epilepsy: why are patients with absence seizures absent? Prog Brain Res 150:271–286. https://doi.org/10.1016/s0079-6123(05)50020-7
Blumenfeld H (2012) Impaired consciousness in epilepsy. Lancet Neurol 11:814–826. https://doi.org/10.1016/s1474-4422(12)70188-6
Smith S (2005) EEG in the diagnosis, classification, and management of patients with epilepsy. J Neurol Neurosurg Psych 76(Suppl 2):ii2–ii7
Wiest MC, Nicolelis MA (2003) Behavioral detection of tactile stimuli during 7–12 Hz cortical oscillations in awake rats. Nat Neurosci 6:913–914. https://doi.org/10.1038/nn1107
D’Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW (2004) Post-traumatic epilepsy following fluid percussion injury in the rat. Brain 127:304–314. https://doi.org/10.1093/brain/awh038
D’Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW (2005) Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain 128:174–188. https://doi.org/10.1093/brain/awh337
Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH (2002) Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci 22:1480–1495. https://doi.org/10.1523/jneurosci.22-04-01480.2002
Lüttjohann A, van Luijtelaar G (2015) Dynamics of networks during absence seizure’s on- and offset in rodents and man. Front Physiol 6:16. https://doi.org/10.3389/fphys.2015.00016
McCafferty C, David F, Venzi M, Lőrincz ML, Delicata F, Atherton Z, Recchia G, Orban G, Lambert RC, Di Giovanni G, Leresche N, Crunelli V (2018) Cortical drive and thalamic feed-forward inhibition control thalamic output synchrony during absence seizures. Nat Neurosci 21:744–756. https://doi.org/10.1038/s41593-018-0130-4
Treven M, Koenig X, Assadpour E, Gantumur E, Meyer C, Hilber K, Boehm S, Kubista H (2015) The anticonvulsant retigabine is a subtype selective modulator of GABAA receptors. Epilepsia 56:647–657. https://doi.org/10.1111/epi.12950
Walker MC, Kullmann DM (2012) Tonic GABA(A) receptor-mediated signaling in epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies. National Center for Biotechnology Information (US), Bethesda, MD
Cope DW, Di Giovanni G, Fyson SJ, Orbán G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V (2009) Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med 15:1392–1398. https://doi.org/10.1038/nm.2058
Pitkänen A, Kharatishvili I, Karhunen H, Lukasiuk K, Immonen R, Nairismägi J, Gröhn O, Nissinen J (2007) Epileptogenesis in experimental models. Epilepsia 48(Suppl 2):13–20. https://doi.org/10.1111/j.1528-1167.2007.01063.x
Hicks R, Soares H, Smith D, McIntosh T (1996) Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol 91:236–246. https://doi.org/10.1007/s004010050421
Hill-Felberg SJ, McIntosh TK, Oliver DL, Raghupathi R, Barbarese E (1999) Concurrent loss and proliferation of astrocytes following lateral fluid percussion brain injury in the adult rat. J Neurosci Res 57:271–279. https://doi.org/10.1002/(sici)1097-4547(19990715)57:2%3c271::aid-jnr13%3e3.0.co;2-z
Jones AL, Britton JW, Blessing MM, Parisi JE, Cascino GD (2018) Chronic traumatic encephalopathy in an epilepsy surgery cohort: Clinical and pathologic findings. Neurology 90:e474–e478. https://doi.org/10.1212/wnl.0000000000004927
Cervós-Navarro J, Lafuente JV (1991) Traumatic brain injuries: structural changes. J Neurol Sci 103(Suppl):S3-14. https://doi.org/10.1016/0022-510x(91)90002-o
Santhakumar V, Aradi I, Soltesz I (2005) Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. J Neurophysiol 93:437–453. https://doi.org/10.1152/jn.00777.2004
Sick T, Wasserman J, Bregy A, Sick J, Dietrich WD, Bramlett HM (2017) Increased expression of epileptiform spike/wave discharges one year after mild, moderate, or severe fluid percussion brain injury in rats. J Neurotrauma 34:2467–2474. https://doi.org/10.1089/neu.2016.4826
Sofroniew MV (2020) Astrocyte reactivity: subtypes, states, and functions in CNS innate immunity. Trends Immunol 41:758–770. https://doi.org/10.1016/j.it.2020.07.004
Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50:427–434. https://doi.org/10.1002/glia.20207
Acknowledgements
We thank Sarina Iwabuchi, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing a draft of this manuscript.
Funding
This work was mainly supported by the startup fund from Capital Medical University Advanced Innovation Center for Human Brain Protection (20181101, JW), and partially by grants from National Key R&D Program of China (2017YFC1307501, QW), Beijing-Tianjin-Hebei Cooperative Basic Research Program (H2018206435, QW), the National Natural Science Foundation of China (81870935, JW).
Author information
Authors and Affiliations
Contributions
LS and RL conducted literature review and wrote the initial draft of the manuscript. HY and TYmade preliminary revision. JW and QW made critical revision. All authors contributed to manuscript revision and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval
All procedures were approved by the Animal Care and Use Committee of Capital Medical University (Beijing, China).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, L., Liu, R., Yang, H. et al. Characteristics of Epileptiform Spike-wave Discharges and Chronic Histopathology in Controlled Cortical Impact Model of Sprague–Dawley Rats. Neurochem Res 47, 3615–3626 (2022). https://doi.org/10.1007/s11064-022-03542-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03542-y