Abstract
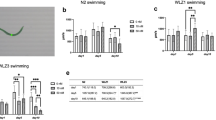
Methylmercury (MeHg) exposure and its harmful effects on the developing brain continue to be a global environmental health concern. Decline in mitochondrial function is central to the toxic effects of MeHg and pathogenesis of mitochondria-related diseases including Parkinson's disease (PD). LRRK2 (Leucine-rich repeat kinase 2) mutation is one of the most common genetic risk factors for PD. In this study, we utilize an acute toxicity model of MeHg exposure in the model organism Caenorhabditis elegans (C. elegans) to compare lifespan, developmental progression, mitochondrial membrane potential and reactive oxygen species (ROS) between the wild-type N2 strain, wild-type LRRK2 transgenic strain (WLZ1), and mutant LRRK2(G2019S) transgenic strain (WLZ3). Additionally, the expression levels of skn-1 and gst-4 were investigated. Our results show that acute MeHg exposure (5 and 10 µM) caused a significant developmental delay in the N2 and WLZ3 worms. Notably, the worms expressing wild-type LRRK2 were resistant to 5 µM MeHg- induced developmental retardation. ROS levels in response to MeHg exposure were increased in the N2 worms, but not in the WLZ1 or WLZ3 worms. The mitochondrial membrane potential was decreased in the N2 worms but increased in the WLZ1 and WLZ3 worms following MeHg exposure. Furthermore, MeHg exposure increased the expression of skn-1 in N2, but not in WLZ1 worms. Although skn-1 expression was increased in the WLZ3 worms following MeHg exposure, gst-4 expression was not induced. Both skn-1 and gst-4 had higher basal expression levels in LRRK2s transgenic than wild-type N2 worms. Knocking down of skn-1 with feeding RNAi had a significant developmental effect in WLZ1 worms; however, the effect was not found in WLZ3 worms. These results suggest that mitochondrial dysfunction and a defect in the SKN-1 signaling in the LRRK2 G2019S worms contribute to the severe developmental delay, establishing a modulatory role of LRRK2 mutation in MeHg-induced acute toxicity.








Similar content being viewed by others
References
Sheehan MC, Burke TA, Navas-Acien A, Breysse PN, McGready J, Fox MA (2014) Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: a systematic review. Bull World Health Organ 92:254–269f
Aschner M, Clarkson TW (1989) Methyl mercury uptake across bovine brain capillary endothelial cells in vitro: the role of amino acids. Pharmacol Toxicol 64:293–297
Aschner M, Eberle NB, Goderie S, Kimelberg HK (1990) Methylmercury uptake in rat primary astrocyte cultures: the role of the neutral amino acid transport system. Brain Res 521:221–228
Pacyna JM, Sundseth K, Pacyna EG, Jozewicz W, Munthe J, Belhaj M, Aström S (2010) An assessment of costs and benefits associated with mercury emission reductions from major anthropogenic sources. J Air Waste Manag Assoc 60:302–315
Schartup AT, Thackray CP, Qureshi A, Dassuncao C, Gillespie K, Hanke A, Sunderland EM (2019) Climate change and overfishing increase neurotoxicant in marine predators. Nature 572:648–650
Myers GJ, Davidson PW (1998) Prenatal methylmercury exposure and children: neurologic, developmental, and behavioral research. Environ Health Perspect 106(Suppl 3):841–847
Davidson PW, Myers GJ, Weiss B, Shamlaye CF, Cox C (2006) Prenatal methyl mercury exposure from fish consumption and child development: a review of evidence and perspectives from the Seychelles Child Development Study. Neurotoxicology 27:1106–1109
Weiss B, Clarkson TW, Simon W (2002) Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ Health Perspect 110(Suppl 5):851–854
Roos D, Seeger R, Puntel R, Vargas Barbosa N (2012) Role of calcium and mitochondria in MeHg-mediated cytotoxicity. J Biomed Biotechnol 2012:248764
Nesci S, Trombetti F, Pirini M, Ventrella V, Pagliarani A (2016) Mercury and protein thiols: stimulation of mitochondrial F(1)F(O)-ATPase and inhibition of respiration. Chem Biol Interact 260:42–49
Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO, Sidoryk M, Albrecht J, Aschner M (2007) Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res 1131:1–10
Ke T, Aschner MJN (2019) Bacteria affect Caenorhabditis elegans responses to MeHg toxicity. Neurotoxicology 75:129–135
LeBel CP, Ali SF, McKee M, Bondy SC (1990) Organometal-induced increases in oxygen reactive species: the potential of 2’,7’-dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol Appl Pharmacol 104:17–24
Mori N, Yasutake A, Marumoto M, Hirayama K (2011) Methylmercury inhibits electron transport chain activity and induces cytochrome c release in cerebellum mitochondria. J Toxicol Sci 36:253–259
Barbosa NV, Nogueira CW, Nogara PA, de Bem AF, Aschner M, Rocha JBT (2017) Organoselenium compounds as mimics of selenoproteins and thiol modifier agents. Metallomics 9:1703–1734
Farina M, Aschner M, Rocha JB (2011) Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol 256:405–417
Ke T, Bornhorst J, Schwerdtle T, Santamaría A, Soare FAA, Rocha JB, Farina M, Bowman AB, Aschner MJNR (2020) Therapeutic Efficacy of the N, N′ Bis-(2-Mercaptoethyl) Isophthalamide Chelator for Methylmercury Intoxication in Caenorhabditis elegans.1–12
Caito SW, Zhang Y, Aschner M (2013) Involvement of AAT transporters in methylmercury toxicity in Caenorhabditis elegans. Biochem Biophys Res Commun 435:546–550
Vanduyn N, Settivari R, Wong G, Nass R (2010) SKN-1/Nrf2 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of methylmercury toxicity. Toxicol Sci 118:613–624
Unoki T, Akiyama M, Kumagai Y, Gonçalves FM, Farina M, da Rocha JBT, Aschner M (2018) Molecular pathways associated with methylmercury-induced Nrf2 modulation. Front Genet 9:373
Ni M, Li X, Yin Z, Jiang H, Sidoryk-Wegrzynowicz M, Milatovic D, Cai J, Aschner M (2010) Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol Sci 116:590–603
Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39:199–218
Bento-Pereira C, Dinkova-Kostova AT (2020) Activation of transcription factor Nrf2 to counteract mitochondrial dysfunction in Parkinson’s disease. Med Res Rev 41(2):785–802
Vorojeikina D, Broberg K, Love TM, Davidson PW, van Wijngaarden E, Rand MD (2017) Editor’s highlight: glutathione S-transferase activity moderates methylmercury toxicity during development in Drosophila. Toxicol Sci 157:211–221
Ke T, Santamaria A, Rocha JBT, Tinkov AA, Lu R, Bowman AB, Aschner M (2020) The role of human LRRK2 in methylmercury-induced inhibition of microvesicle formation of cephalic neurons in Caenorhabditis elegans. Neurotoxicol Res 38:751–764
Tolosa E, Vila M, Klein C, Rascol O (2020) LRRK2 in Parkinson disease: challenges of clinical trials. Nat Rev Neurol 16:97–107
Howlett EH, Jensen N, Belmonte F, Zafar F, Hu X, Kluss J, Schule B, Kaufman BA, Greenamyre JT, Sanders LH (2017) LRRK2 G2019S-induced mitochondrial DNA damage is LRRK2 kinase dependent and inhibition restores mtDNA integrity in Parkinson’s disease. Hum Mol Genet 26:4340–4351
Saha S, Guillily MD, Ferree A, Lanceta J, Chan D, Ghosh J, Hsu CH, Segal L, Raghavan K, Matsumoto K, Hisamoto N, Kuwahara T, Iwatsubo T, Moore L, Goldstein L, Cookson M, Wolozin B (2009) LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci 29:9210–9218
Loeffler DA, Klaver AC, Coffey MP, Aasly JO, LeWitt PA (2017) Increased oxidative stress markers in cerebrospinal fluid from healthy subjects with Parkinson’s disease-associated LRRK2 gene mutations. Front Aging Neurosci 9:89
Lionaki E, Markaki M, Palikaras K, Tavernarakis N (2015) Mitochondria, autophagy and age-associated neurodegenerative diseases: new insights into a complex interplay. Biochim Biophys Acta 1847:1412–1423
Ke T, Antunes Soares FA, Santamaría A, Bowman AB, Skalny AV, Aschner M (2020) N, N’ bis-(2-mercaptoethyl) isophthalamide induces developmental delay in Caenorhabditis elegans by promoting DAF-16 nuclear localization. Toxicol Rep 7:930–937
Ke T, Tsatsakis A, Santamaría A, Soare FAA, Tinkov AA, Docea AO, Skalny A, Bowman AB, Aschner MJN (2020) Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans. Neurotoxicology 7:105–113
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25:402–408
Shabalina IG, Nedergaard J (2011) Mitochondrial ('mild’) uncoupling and ROS production: physiologically relevant or not? Biochem Soc Trans 39:1305–1309
Hare MF, Atchison WD (1992) Comparative action of methylmercury and divalent inorganic mercury on nerve terminal and intraterminal mitochondrial membrane potentials. J Pharmacol Exp Ther 261:166–172
Papkovskaia TD, Chau KY, Inesta-Vaquera F, Papkovsky DB, Healy DG, Nishio K, Staddon J, Duchen MR, Hardy J, Schapira AH, Cooper JM (2012) G2019S leucine-rich repeat kinase 2 causes uncoupling protein-mediated mitochondrial depolarization. Hum Mol Genet 21:4201–4213
Mortiboys H, Johansen KK, Aasly JO, Bandmann O (2010) Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology 75:2017–2020
Scorrano L, Petronilli V, Bernardi P (1997) On the voltage dependence of the mitochondrial permeability transition pore. A critical appraisal. J Biol Chem 272:12295–12299
Duchen MR (2000) Mitochondria and calcium: from cell signalling to cell death. J Physiol 529(Pt 1):57–68
Levesque PC, Atchison WD (1991) Disruption of brain mitochondrial calcium sequestration by methylmercury. J Pharmacol Exp Ther 256:236–242
Padalko VI (2005) Uncoupler of oxidative phosphorylation prolongs the lifespan of Drosophila. Biochemistry (Mosc) 70:986–989
Marty MS, Atchison WD (1997) Pathways mediating Ca2+ entry in rat cerebellar granule cells following in vitro exposure to methyl mercury. Toxicol Appl Pharmacol 147:319–330
Verma M, Callio J, Otero PA, Sekler I, Wills ZP, Chu CT (2017) Mitochondrial calcium dysregulation contributes to dendrite degeneration mediated by PD/LBD-associated LRRK2 mutants. J Neurosci 37:11151–11165
Delcambre S, Ghelfi J, Ouzren N, Grandmougin L, Delbrouck C, Seibler P, Wasner K, Aasly JO, Klein C, Trinh J, Pereira SL, Grünewald A (2020) Mitochondrial mechanisms of LRRK2 G2019S penetrance. Front Neurol 11:881
Acknowledgements
This work was supported by the National Institutes of Health to MA and ABB (NIEHS R01ES007331). Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ke, T., Rocha, J.B.T., Tinkov, A.A. et al. The Role of Human LRRK2 in Acute Methylmercury Toxicity in Caenorhabditis elegans. Neurochem Res 46, 2991–3002 (2021). https://doi.org/10.1007/s11064-021-03394-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03394-y