Abstract
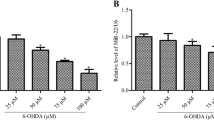
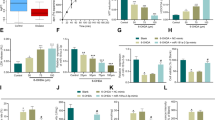
MicroRNA-33-3p (miR-33-3p) has been widely investigated for its roles in lipid metabolism and mitochondrial function; however, there are few studies on miR-33-3p in the context of neurological diseases. In this study, we investigated the functional role of miR-33-3p in rat pheochromocytoma PC12 cells. A miR-33-3p mimic was transduced into PC12 cells, and its effects on proliferation, apoptosis, and differentiation were studied using the MTS assay, EdU labeling, flow cytometry, qRT-PCR, western blot, ELISA, and immunofluorescence. We found that miR-33-3p significantly suppressed PC12 cell proliferation, but had no effect on apoptosis. Furthermore, miR-33-3p promoted the differentiation of PC12 cells into Tuj1-positive and choline acetyltransferase-positive neuron-like cells. Mechanistically, miR-33-3p repressed the expression of Slc29a1 in PC12 cells. Importantly, knocking down Slc29a1 in PC12 cells inhibited proliferation and induced differentiation into neuron-like cells. In conclusion, this study showed that miR-33-3p regulated Slc29a1, which in turn controlled the proliferation and differentiation of PC12 cells. Thus, we hypothesize that the miR-33-3p/Slc29a1 axis could be a promising therapeutic target for recovering neurons and the cholinergic nervous system.





Similar content being viewed by others
References
Hashemiaghdam A, Mroczek M (2020) Microglia heterogeneity and neurodegeneration: the emerging paradigm of the role of immunity in Alzheimer’s disease. J Neuroimmunol 341:577185
Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25:59–70
Haam J, Yakel JL (2017) Cholinergic modulation of the hippocampal region and memory function. J Neurochem 142(Suppl 2):111–121
Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, Khachaturian ZS (2018) The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141:1917–1933
Conti I, Varano G, Simioni C, Laface I, Milani D, Rimondi E, Neri LM (2020) miRNAs as influencers of cell–cell communication in tumor microenvironment. Cells 9:220
Olejniczak M, Kotowska-Zimmer A, Krzyzosiak W (2018) Stress-induced changes in miRNA biogenesis and functioning. Cell Mol Life Sci 75:177–191
Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM, Voinea SC (2020) miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells 9:276
Aryal B, Singh AK, Rotllan N, Price N, Fernandez-Hernando C (2017) MicroRNAs and lipid metabolism. Curr Opin Lipidol 28:273–280
Ouimet M, Ediriweera H, Afonso MS, Ramkhelawon B, Singaravelu R, Liao X, Bandler RC, Rahman K, Fisher EA, Rayner KJ, Pezacki JP, Tabas I, Moore KJ (2017) microRNA-33 regulates macrophage autophagy in atherosclerosis. Arterioscler Thromb Vasc Biol 37:1058–1067
Price NL, Miguel V, Ding W, Singh AK, Malik S, Rotllan N, Moshnikova A, Toczek J, Zeiss C, Sadeghi MM, Arias N, Baldan A, Andreev OA, Rodriguez-Puyol D, Bahal R, Reshetnyak YK, Suarez Y, Fernandez-Hernando C, Lamas S (2019) Genetic deficiency or pharmacological inhibition of miR-33 protects from kidney fibrosis. JCI Insight 4:22
Wang H, Sun Z, Wang Y, Hu Z, Zhou H, Zhang L, Hong B, Zhang S, Cao X (2016) miR-33-5p, a novel mechano-sensitive microRNA promotes osteoblast differentiation by targeting Hmga2. Sci Rep 6:23170
Price NL, Fernandez-Hernando C (2015) Novel role of miR-33 in regulating of mitochondrial function. Circ Res 117:225–228
Jiang J, Bi Y, Liu XP, Yu D, Yan X, Yao J, Liu T, Li S (2020) To construct a ceRNA regulatory network as prognostic biomarkers for bladder cancer. J Cell Mol Med 24:5375–5386
Peng W, Li J, Chen R, Gu Q, Yang P, Qian W, Ji D, Wang Q, Zhang Z, Tang J, Sun Y (2019) Upregulated METTL3 promotes metastasis of colorectal cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res 38:393
da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57
Kabekkodu SP, Shukla V, Varghese VK, D’Souza J, Chakrabarty S, Satyamoorthy K (2018) Clustered miRNAs and their role in biological functions and diseases. Biol Rev Camb Philos Soc 93:1955–1986
Beermann J, Piccoli M-T, Viereck J, Thum T (2016) Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev 96:1297–1325
Fabian MR, Sonenberg N, Filipowicz W (2010) Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 79:351–379
Näär AM (2018) miR-33: a metabolic conundrum. Trends Endocrinol Metab 29:667–668
Aryal B, Singh AK, Rotllan N, Price N, Fernández-Hernando C (2017) MicroRNAs and lipid metabolism. Curr Opin Lipidol 28:273–280
Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, Rinehold K, van Solingen C, Fullerton MD, Cecchini K, Rayner KJ, Steinberg GR, Zamore PD, Fisher EA, Pn L, Moore KJ (2015) MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest 125:4334–4348
Hangya B, Ranade SP, Lorenc M, Kepecs A (2015) Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell 162:1155–1168
Joshi A, Kalappa BI, Anderson CT, Tzounopoulos T (2016) Cell-specific cholinergic modulation of excitability of layer 5B principal neurons in mouse auditory cortex. J Neurosci 36:8487–8499
Colangelo C, Shichkova P, Keller D, Markram H, Ramaswamy S (2019) Cellular, synaptic and network effects of acetylcholine in the neocortex. Front Neural Circuits 13:24
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14:101–115
de Medeiros LM, De Bastiani MA, Rico EP, Schonhofen P, Pfaffenseller B, Wollenhaupt-Aguiar B, Grun L, Barbe-Tuana F, Zimmer ER, Castro MAA, Parsons RB, Klamt F (2019) Cholinergic differentiation of human neuroblastoma SH-SY5Y cell line and its potential use as an in vitro model for Alzheimer’s disease studies. Mol Neurobiol 56:7355–7367
Ztaou S, Amalric M (2019) Contribution of cholinergic interneurons to striatal pathophysiology in Parkinson’s disease. Neurochem Int 126:1–10
Vlachodimou A, Konstantinopoulou K, Ijzerman AP, Heitman LH (2020) Affinity, binding kinetics and functional characterization of draflazine analogues for human equilibrative nucleoside transporter 1 (SLC29A1). Biochem Pharmacol 172:113747
Wright NJ, Lee S-Y (2019) Structures of human ENT1 in complex with adenosine reuptake inhibitors. Nat Struct Mol Biol 26:599–606
Lee C-C, Chang C-P, Lin C-J, Lai H-L, Kao Y-H, Cheng S-J, Chen H-M, Liao Y-P, Faivre E, Buée L, Blum D, Fang J-M, Chern Y (2018) Adenosine augmentation evoked by an ENT1 inhibitor improves memory impairment and neuronal plasticity in the APP/PS1 mouse model of Alzheimer’s disease. Mol Neurobiol 55:8936–8952
Zhang D, Jin W, Liu H, Liang T, Peng Y, Zhang J, Zhang Y (2020) ENT1 inhibition attenuates apoptosis by activation of cAMP/pCREB/Bcl2 pathway after MCAO in rats. Exp Neurol 331:113362
Acknowledgements
We gratefully acknowledge the Graduate Scientific Research Innovation Program of Jiangsu Province, Natural Science Foundation of Jiangsu Province and Nantong University, and a Project Funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education institutions for the research grants. We also acknowledge the editor and reviewers for their helpful comments on this paper.
Funding
Contract grant sponsor: Graduate Scientific Research Innovation Program of Jiangsu Province; Contract Grant Number: KYCX19 2066. Contract grant sponsor: Natural Science Foundation of Jiangsu Province; Contract Grant Number: BK20170447. Contract grant sponsor: a Project Funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education institutions. Contract Grant Number: 03081023.
Author information
Authors and Affiliations
Contributions
BS, and WL conceived the experiments. BS performed the experimenets with help from HZ, MT, XC, JQ. BS and GJ analyzed the data. BS, JQ, and GJ wrote the manuscript. All authors read, revised, and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
We have no conflicts of interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 254 KB)
Fig. S1 Interference efficiency of Slc29a1 was detected by qRT-PCR and Western blot.
Supplementary file2 (PDF 2236 KB)
Fig. S2 Effects of miR-33-3p-Slc29a1 on NSCs differentiation. a,b The concentration of ACh and ChAT level were detected with ELISA assay. c,d Immunocytochemical analyse was applied to detected he percentage of Tuj1 (red) and ChA T(red) immunopositive cells. Nuclei are visualized with Hoechst (blue), and analyzed by fluorescent microscopy. Scale bar, 100 μm. Data are expressed as the mean ± SD; n = 3. **P<0.01, ***P<0.001.
Rights and permissions
About this article
Cite this article
Shan, BQ., Li, W., He, H. et al. miR-33-3p Regulates PC12 Cell Proliferation and Differentiation In Vitro by Targeting Slc29a1. Neurochem Res 46, 2403–2414 (2021). https://doi.org/10.1007/s11064-021-03377-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03377-z