Abstract
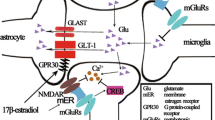
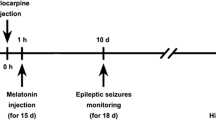
G-protein coupled estrogen receptor 1 (GPER1) is a novel type of estrogen receptor. Several studies have shown that it has an anti-inflammatory action,which plays an important role in remyelination and cognitive ability adjustment. However, whether it is involved in the development of temporal lobe epilepsy (TLE) is still unknown. The present study established a TLE model by intraperitoneal injection of lithium chloride (3 mmol/kg) and pilocarpine (50 mg/kg) in rats to study the effect of GPER1 in the synaptic plasticity during the development of temporal lobe epilepsy. A microinjection cannula was implanted into the lateral ventricle region of rats via a stereotaxic instrument. G-1 is the specific GPER1 agonist and G15 is the specific GPER1 antagonist. The G1 or G15 and Dimethyl sulfoxide were injected into the rat brains in the intervention groups and control group, respectively. After G1 intervention, the learning and memory abilities and hippocampal neuron damage in epileptic rats were significantly improved, while G15 weakened the neuroprotective effect of GPER1. Meanwhile, G1 controlled the abnormal formation of hippocampal mossy fiber sprouting caused by seizures, and participated in the regulation of synaptic plasticity by reducing the expression of Synapsin I and increasing the expression of gephyrin. Inhibitory synapse gephyrin may play a significant role in synaptic plasticity.






Similar content being viewed by others
Abbreviations
- GPER1:
-
G protein-coupled estrogen receptor 1
- TLE:
-
Temporal lobe epilepsy
- SE:
-
Status epilepticus
- DMSO:
-
Dimethyl sulfoxide
- MFS:
-
Mossy fiber sprouting
References
Akama KT, Thompson LI, Milner TA, McEwen BS (2013) Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J Biol Chem 288:6438–6450
Alexander A, Irving AJ, Harvey J (2017) Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology 113:652–660
Kumar S, Singh MB, Shukla G, Vishnubhatla S, Srivastava MVP, Goyal V, Prasad K, Patterson V (2017) Effective clinical classification of chronic epilepsy into focal and generalized: a cross sectional study. Seizure 53:81–85
Temkin NR (2001) Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia 42:515–524
Prossnitz ER, Maggiolini M (2009) Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol 308:32–38
Mendes-Oliveira J, Lopes Campos F, Videira RA, Baltazar G (2017) GPER activation is effective in protecting against inflammation-induced nigral dopaminergic loss and motor function impairment. Brain Behav Immun 64:296–307
Hirahara Y, Matsuda KI, Yamada H, Saitou A, Morisaki S, Takanami K, Boggs JM, Kawata M (2013) G protein-coupled receptor 30 contributes to improved remyelination after cuprizone-induced demyelination. Glia 61:420–431
Kurt AH, Bosnak M, Inan SY, Celik A, Uremis MM (2016) Epileptogenic effects of G protein-coupled estrogen receptor 1 in the rat pentylenetetrazole kindling model of epilepsy. Pharmacol Rep 68:66–70
Zuo D, Wang F, Rong W, Wen Y, Sun K, Zhao X, Ren X, He Z, Ding N, Ma L, Xu F (2020) The novel estrogen receptor GPER1 decreases epilepsy severity and susceptivity in the hippocampus after status epilepticus. Neurosci Lett 728:134978
Iravani M, Lagerquist MK, Karimian E, Chagin AS, Ohlsson C, Savendahl L (2019) Effects of the selective GPER1 agonist G1 on bone growth. Endocr Connect 8:1302–1309
Liu C, Liao Y, Fan S, Fu X, Xiong J, Zhou S, Zou M, Wang J (2019) G-protein-coupled estrogen receptor antagonist G15 decreases estrogen-induced development of non-small cell lung cancer. Oncol Res 27:283–292
Wang XS, Yue J, Hu LN, Tian Z, Zhang K, Yang L, Zhang HN, Guo YY, Feng B, Liu HY, Wu YM, Zhao MG, Liu SB (2020) Activation of G protein-coupled receptor 30 protects neurons by regulating autophagy in astrocytes. Glia 68:27–43
Hart D, Nilges M, Pollard K, Lynn T, Patsos O, Shiel C, Clark SM, Vasudevan N (2014) Activation of the G-protein coupled receptor 30 (GPR30) has different effects on anxiety in male and female mice. Steroids 81:49–56
Kim TY, Yi JS, Chung SJ, Kim DK, Byun HR, Lee JY, Koh JY (2007) Pyruvate protects against kainate-induced epileptic brain damage in rats. Exp Neurol 208:159–167
Bailey CH, Kandel ER, Harris KM (2015) Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb Perspect Biol 7:a021758
Lin H, Huang Y, Wang Y, Jia J (2011) Spatiotemporal profile of N-cadherin expression in the mossy fiber sprouting and synaptic plasticity following seizures. Mol Cell Biochem 358:201–205
Rezende GH, Guidine PA, Medeiros Dde C, Moraes-Santos T, Mello LE, Moraes MF (2015) Protein-caloric dietary restriction inhibits mossy fiber sprouting in the pilocarpine model of TLE without significantly altering seizure phenotype. Epilepsy Res 117:85–89
Bonanomi D, Pennuto M, Rigoni M, Rossetto O, Montecucco C, Valtorta F (2005) Taipoxin induces synaptic vesicle exocytosis and disrupts the interaction of synaptophysin I with VAMP2. Mol Pharmacol 67:1901–1908
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Kim JE, Kim YJ, Lee DS, Kim JY, Ko AR, Hyun HW, Kim MJ, Kang TC (2016) PLPP/CIN regulates bidirectional synaptic plasticity via GluN2A interaction with postsynaptic proteins. Sci Rep 6:26576
Ma J, Wang J, Lv C, Pang J, Han B, Wang M, Gen Y (2017) The role of hippocampal structural synaptic plasticity in repetitive transcranial magnetic stimulation to improve cognitive function in male SAMP8 mice. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 41:137–44
Nikolaev M, Heggelund P (2015) Functions of synapsins in corticothalamic facilitation: important roles of Synapsin I. J Physiol 593:4499–4510
Sun J, Bronk P, Liu X, Han W, Sudhof TC (2006) Synapsins regulate use-dependent synaptic plasticity in the calyx of Held by a Ca2+/calmodulin-dependent pathway. Proc Natl Acad Sci USA 103:2880–2885
Jacob TC, Moss SJ, Jurd R (2008) GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci 9:331–343
Nawrotzki R, Islinger M, Vogel I, Volkl A, Kirsch J (2012) Expression and subcellular distribution of gephyrin in non-neuronal tissues and cells. Histochem Cell Biol 137:471–482
Zacchi P, Antonelli R, Cherubini E (2014) Gephyrin phosphorylation in the functional organization and plasticity of GABAergic synapses. Front Cell Neurosci 8:103
Song P, Liu Y, Yu X, Wu J, Poon AN, Demaio A, Wang W, Rudan I, Chan KY (2017) Prevalence of epilepsy in China between 1990 and 2015: a systematic review and meta-analysis. J Global Health 7:020706
Busch RM, Hogue O, Kattan MW, Hamberger M, Drane DL, Hermann B, Kim M, Ferguson L, Bingaman W, Gonzalez-Martinez J, Najm IM, Jehi L (2018) Nomograms to predict naming decline after temporal lobe surgery in adults with epilepsy. Neurology 91:e2144–e2152
Uribe-San-Martin R, Ciampi E, Di Giacomo R, Vasquez M, Carcamo C, Godoy J, Lo Russo G, Tassi L (2018) Corpus callosum atrophy and post-surgical seizures in temporal lobe epilepsy associated with hippocampal sclerosis. Epilepsy Res 142:29–35
Chun E, Bumanglag AV, Burke SN, Sloviter RS (2019) Targeted hippocampal GABA neuron ablation by stable substance P-saporin causes hippocampal sclerosis and chronic epilepsy in rats. Epilepsia 60:e52–e57
Thomazeau A, Bosch M, Essayan-Perez S, Barnes SA, De Jesus-Cortes H, Bear MF (2020) Dissociation of functional and structural plasticity of dendritic spines during NMDAR and mGluR-dependent long-term synaptic depression in wild-type and fragile X model mice. Mol Psychiatry. https://doi.org/10.1038/s41380-020-0821-6
Hamidi N, Nozad A, Sheikhkanloui Milan H, Amani M (2019) Okadaic acid attenuates short-term and long-term synaptic plasticity of hippocampal dentate gyrus neurons in rats. Neurobiol Learn Mem 158:24–31
Ma T, Cheng Y, Roltsch Hellard E, Wang X, Lu J, Gao X, Huang CCY, Wei XY, Ji JY, Wang J (2018) Bidirectional and long-lasting control of alcohol-seeking behavior by corticostriatal LTP and LTD. Nat Neurosci 21:373–383
Pietras B, Devalle F, Roxin A, Daffertshofer A, Montbrio E (2019) Exact firing rate model reveals the differential effects of chemical versus electrical synapses in spiking networks. Phys Rev E 100:042412
Hawley WR, Grissom EM, Moody NM, Dohanich GP, Vasudevan N (2014) Activation of G-protein-coupled receptor 30 is sufficient to enhance spatial recognition memory in ovariectomized rats. Behav Brain Res 262:68–73
Chen XM, Ji SF, Liu YH, Xue XM, Xu J, Gu ZH, Deng SL, Liu CD, Wang H, Chang YM, Wang XC (2020) Ginsenoside Rd ameliorates auditory cortex injury associated with military aviation noise-induced hearing loss by activating SIRT1/PGC-1alpha signaling pathway. Front Physiol 11:788
Chandrasekaran K, Choi J, Arvas MI, Salimian M, Singh S, Xu S, Gullapalli RP, Kristian T, Russell JW (2020) Nicotinamide mononucleotide administration prevents experimental diabetes-induced cognitive impairment and loss of hippocampal neurons. Int J Mol Sci 21:3756
Cavarsan CF, Malheiros J, Hamani C, Najm I, Covolan L (2018) Is mossy fiber sprouting a potential therapeutic target for epilepsy? Front Neurol 9:1023
Ginsberg SD, Maesako M, Zoltowska KM, Berezovska O (2019) Synapsin 1 promotes Aβ generation via BACE1 modulation. PloS One 14:e0226368
Lionel AC, Vaags AK, Sato D, Gazzellone MJ, Mitchell EB, Chen HY, Costain G, Walker S, Egger G, Thiruvahindrapuram B, Merico D, Prasad A, Anagnostou E, Fombonne E, Zwaigenbaum L, Roberts W, Szatmari P, Fernandez BA, Georgieva L, Brzustowicz LM, Roetzer K, Kaschnitz W, Vincent JB, Windpassinger C, Marshall CR, Trifiletti RR, Kirmani S, Kirov G, Petek E, Hodge JC, Bassett AS, Scherer SW (2013) Rare exonic deletions implicate the synaptic organizer Gephyrin (GPHN) in risk for autism, schizophrenia and seizures. Hum Mol Genet 22:2055–2066
Acknowledgements
The authors would like to acknowledge the Ningxia Medical University and Ningxia Hui Autonomous Region to support this work.
Funding
This work was supported by The 13.5 Major Scientific and Technological Projects of the Ningxia Hui Autonomous Region [Grant No. 2016BZ07], Ningxia Natural Science Foundation [Grant No. NZ17069], and Ningxia Medical University Advantage Discipline Group Project [Grant No. XY201902].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11064_2021_3336_MOESM1_ESM.tif
Fig. S. 1 The expression of GPER1 in hippocampus CA3 and DG regions in rats per group after intervention. (a) Immunohistochemistry in rats per group, (b) The expression of GPER1 in each group of rats; scale bar = 20 μm. Supplementary material 1 (TIF 20883.0 kb)
11064_2021_3336_MOESM2_ESM.tif
Fig. S. 2 Nissl staining was used to detect the damage and repair of CA3 and DG regions’ hippocampal neurons per group in rats; scale bar = 20 μm. Supplementary material 2 (TIF 23375.1 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Yang, Y., Guo, L. et al. GPER1 Modulates Synaptic Plasticity During the Development of Temporal Lobe Epilepsy in Rats. Neurochem Res 46, 2019–2032 (2021). https://doi.org/10.1007/s11064-021-03336-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03336-8