Abstract
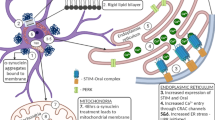
Alpha-synuclein plays a vital role in the pathology of Parkinson’s disease (PD). Spreading of α-synuclein in neighboring cells was believed to contribute to progression in PD. How α-synuclein transmission affects adjacent cells is not full elucidated. Here, we used recombinant α-synuclein to mimic intercellular transmitted α-synuclein in MES23.5 dopaminergic cells, to investigate whether and how it could modulate iron metabolism. The results showed that α-synuclein treatment up-regulated divalent metal transporter 1 (DMT1) and down-regulated iron transporter (FPN), also up-regulated iron regulatory protein 1 (IRP1) protein levels and hepcidin mRNA levels. Endocytosis inhibitor dynasore pretreatment completely abolished and even reversed the upregulation of DMT1 and IRP1 induced by α-synuclein, however, FPN down-regulation was partially blocked by dynasore. Autophagy-inducing agent rapamycin reversed DMT1 up-regulation and FPN down-regulation, and fully blocked the upregulation of IRP1. Elevated hepcidin levels induced by α-synuclein was fully blocked by dynasore pretreatment, however, even higher with rapamycin pretreatment. Alpha-synuclein treatment triggered endoplasmic reticulum (ER) stress. ER stress inducer thapsigargin induced similar responses elicited by α-synuclein. ER stress inhibitor salubrinal blocked the up-regulation of IRP1 and hepcidin, as well as DMT1 up-regulation and FPN down-regulation, also dramatically abolished cAMP-response elements binding protein phosphorylation induced by α-synuclein. Taken together, these finding indicated that extracellular α-synuclein could regulate cellular iron metabolism, probably mediated by ER stress. It provides novel evidence to elucidate the relationships between transmitted α-synuclein and iron metabolism disturbance in PD.







Similar content being viewed by others
References
Przedborski S (2007) Neuroinflammation and Parkinson’s disease. Handb Clin Neurol 83:535–551. https://doi.org/10.1016/s0072-9752(07)83026-0
Schon EA, Przedborski S (2011) Mitochondria: the next (neurode)generation. Neuron 70(6):1033–1053. https://doi.org/10.1016/j.neuron.2011.06.003
Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14(1):38–48. https://doi.org/10.1038/nrn3406
Przedborski S (2017) The two-century journey of Parkinson disease research. Nat Rev Neurosci 18(4):251–259. https://doi.org/10.1038/nrn.2017.25
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912. https://doi.org/10.1016/s0140-6736(14)61393-3
Mehra S, Sahay S (1867) Maji SK (2019) α-Synuclein misfolding and aggregation: implications in Parkinson’s disease pathogenesis. Biochim Biophys Acta 10:890–908. https://doi.org/10.1016/j.bbapap.2019.03.001
Thayanidhi N, Helm JR, Nycz DC, Bentley M, Liang Y, Hay JC (2010) Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs. Mol Biol Cell 21(11):1850–1863. https://doi.org/10.1091/mbc.e09-09-0801
Villar-Piqué A, Lopes da Fonseca T, Outeiro TF (2016) Structure, function and toxicity of alpha-synuclein: the Bermuda triangle in synucleinopathies. J Neurochem 139(Suppl 1):240–255. https://doi.org/10.1111/jnc.13249
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14(5):504–506. https://doi.org/10.1038/nm1747
Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, Widner H, Revesz T, Lindvall O, Brundin P (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14(5):501–503. https://doi.org/10.1038/nm1746
Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ (2008) Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol 40(9):1835–1849. https://doi.org/10.1016/j.biocel.2008.01.017
Danzer KM, Krebs SK, Wolff M, Birk G, Hengerer B (2009) Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J Neurochem 111(1):192–203. https://doi.org/10.1111/j.1471-4159.2009.06324.x
Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA 106(31):13010–13015. https://doi.org/10.1073/pnas.0903691106
Lee HJ, Bae EJ, Lee SJ (2014) Extracellular α–synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol 10(2):92–98. https://doi.org/10.1038/nrneurol.2013.275
Lee HJ, Patel S, Lee SJ (2005) Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 25(25):6016–6024. https://doi.org/10.1523/jneurosci.0692-05.2005
Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ (2010) Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem 113(5):1263–1274. https://doi.org/10.1111/j.1471-4159.2010.06695.x
Malhotra V (2013) Unconventional protein secretion: an evolving mechanism. EMBO J 32(12):1660–1664. https://doi.org/10.1038/emboj.2013.104
Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30(20):6838–6851. https://doi.org/10.1523/jneurosci.5699-09.2010
Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7:42. https://doi.org/10.1186/1750-1326-7-42
Wu X, Zheng T, Zhang B (2017) Exosomes in Parkinson’s disease. Neurosci Bull 33(3):331–338. https://doi.org/10.1007/s12264-016-0092-z
Stuendl A, Kunadt M, Kruse N, Bartels C, Moebius W, Danzer KM, Mollenhauer B, Schneider A (2016) Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain 139(Pt 2):481–494. https://doi.org/10.1093/brain/awv346
Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Banks WA, Zhang J (2014) Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128(5):639–650. https://doi.org/10.1007/s00401-014-1314-y
Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ (2010) Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 285(12):9262–9272. https://doi.org/10.1074/jbc.M109.081125
Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ (2013) Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun 4:1562. https://doi.org/10.1038/ncomms2534
Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM (2011) Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72(1):57–71. https://doi.org/10.1016/j.neuron.2011.08.033
Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338(6109):949–953. https://doi.org/10.1126/science.1227157
Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G, Youdim MB (1988) Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm 74(3):199–205. https://doi.org/10.1007/bf01244786
Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA, Elstner M, Morris CM (2007) Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology 68(21):1820–1825. https://doi.org/10.1212/01.wnl.0000262033.01945.9a
Jiang H, Wang J, Rogers J, Xie J (2017) Brain iron metabolism dysfunction in Parkinson’s disease. Mol Neurobiol 54(4):3078–3101. https://doi.org/10.1007/s12035-016-9879-1
Zucca FA, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L (2017) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol 155:96–119. https://doi.org/10.1016/j.pneurobio.2015.09.012
Hare DJ, Double KL (2016) Iron and dopamine: a toxic couple. Brain 139(Pt 4):1026–1035. https://doi.org/10.1093/brain/aww022
Ganz T (2005) Cellular iron: ferroportin is the only way out. Cell Metab 1(3):155–157. https://doi.org/10.1016/j.cmet.2005.02.005
Du F, Qian ZM, Luo Q, Yung WH, Ke Y (2015) Hepcidin suppresses brain iron accumulation by downregulating iron transport proteins in iron-overloaded rats. Mol Neurobiol 52(1):101–114. https://doi.org/10.1007/s12035-014-8847-x
Zhang S, Wang J, Song N, Xie J, Jiang H (2009) Up-regulation of divalent metal transporter 1 is involved in 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in MES23.5 cells. Neurobiol Aging 30(9):1466–1476. https://doi.org/10.1016/j.neurobiolaging.2007.11.025
Song N, Wang J, Jiang H, Xie J (2010) Ferroportin 1 but not hephaestin contributes to iron accumulation in a cell model of Parkinson’s disease. Free Radical Biol Med 48(2):332–341. https://doi.org/10.1016/j.freeradbiomed.2009.11.004
Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, González-Billault C, Núñez MT (2013) Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem 126(4):541–549. https://doi.org/10.1111/jnc.12244
De Domenico I, Nemeth E, Nelson JM, Phillips JD, Ajioka RS, Kay MS, Kushner JP, Ganz T, Ward DM, Kaplan J (2014) Retraction notice to: the hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab 19(6):1067. https://doi.org/10.1016/j.cmet.2014.05.010
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306(5704):2090–2093. https://doi.org/10.1126/science.1104742
Castellani RJ, Siedlak SL, Perry G, Smith MA (2000) Sequestration of iron by Lewy bodies in Parkinson’s disease. Acta Neuropathol 100(2):111–114. https://doi.org/10.1007/s004010050001
Chen B, Wen X, Jiang H, Wang J, Song N, Xie J (2019) Interactions between iron and α-synuclein pathology in Parkinson’s disease. Free Radical Biol Med 141:253–260. https://doi.org/10.1016/j.freeradbiomed.2019.06.024
Song N, Xie J (2018) Iron, dopamine, and α-synuclein interactions in at-risk dopaminergic neurons in Parkinson’s disease. Neurosci Bull 34(2):382–384. https://doi.org/10.1007/s12264-018-0209-7
Duce JA, Wong BX, Durham H, Devedjian JC, Smith DP, Devos D (2017) Post translational changes to α-synuclein control iron and dopamine trafficking; a concept for neuron vulnerability in Parkinson’s disease. Mol Neurodegener 12(1):45. https://doi.org/10.1186/s13024-017-0186-8
Xu H, Liu X, Xia J, Yu T, Qu Y, Jiang H, Xie J (2018) Activation of NMDA receptors mediated iron accumulation via modulating iron transporters in Parkinson’s disease. FASEB J. https://doi.org/10.1096/fj.201800060RR
Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, Dawson VL, Dawson TM, Ross CA (2005) Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet 14(24):3801–3811. https://doi.org/10.1093/hmg/ddi396
Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, Schneider BL, Lee MK (2012) Endoplasmic reticulum stress is important for the manifestations of α-synucleinopathy in vivo. J Neurosci 32(10):3306–3320. https://doi.org/10.1523/jneurosci.5367-11.2012
Chung CY, Khurana V, Auluck PK, Tardiff DF, Mazzulli JR, Soldner F, Baru V, Lou Y, Freyzon Y, Cho S, Mungenast AE, Muffat J, Mitalipova M, Pluth MD, Jui NT, Schüle B, Lippard SJ, Tsai LH, Krainc D, Buchwald SL, Jaenisch R, Lindquist S (2013) Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science 342(6161):983–987. https://doi.org/10.1126/science.1245296
Heman-Ackah SM, Manzano R, Hoozemans JJM, Scheper W, Flynn R, Haerty W, Cowley SA, Bassett AR, Wood MJA (2017) Alpha-synuclein induces the unfolded protein response in Parkinson’s disease SNCA triplication iPSC-derived neurons. Hum Mol Genet 26(22):4441–4450. https://doi.org/10.1093/hmg/ddx331
Luo QQ, Qian ZM, Zhou YF, Zhang MW, Wang D, Zhu L, Ke Y (2016) Expression of iron regulatory protein 1 is regulated not only by HIF-1 but also pCREB under hypoxia. Int J Biol Sci 12(10):1191–1202. https://doi.org/10.7150/ijbs.16437
Benskey MJ, Perez RG, Manfredsson FP (2016) The contribution of alpha synuclein to neuronal survival and function: implications for Parkinson’s disease. J Neurochem 137(3):331–359. https://doi.org/10.1111/jnc.13570
Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25(1):239–252. https://doi.org/10.1016/s0896-6273(00)80886-7
De Domenico I, Ward DM, Kaplan J (2011) Hepcidin and ferroportin: the new players in iron metabolism. Semin Liver Dis 31(3):272–279. https://doi.org/10.1055/s-0031-1286058
Bergamaschi G, Di Sabatino A, Pasini A, Ubezio C, Costanzo F, Grataroli D, Masotti M, Alvisi C, Corazza GR (2017) Intestinal expression of genes implicated in iron absorption and their regulation by hepcidin. Clin Nutr 36(5):1427–1433. https://doi.org/10.1016/j.clnu.2016.09.021
Rouault TA (2006) The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol 2(8):406–414. https://doi.org/10.1038/nchembio807
Piccinelli P, Samuelsson T (2007) Evolution of the iron-responsive element. RNA 13(7):952–966. https://doi.org/10.1261/rna.464807
Flamment M, Hajduch E, Ferré P, Foufelle F (2012) New insights into ER stress-induced insulin resistance. Trends Endocrinol Metabol 23(8):381–390. https://doi.org/10.1016/j.tem.2012.06.003
Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA (2001) Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett 509(2):309–316. https://doi.org/10.1016/s0014-5793(01)03189-1
Anderson CP, Shen M, Eisenstein RS (1823) Leibold EA (2012) Mammalian iron metabolism and its control by iron regulatory proteins. Biochem Biophys Acta 9:1468–1483. https://doi.org/10.1016/j.bbamcr.2012.05.010
Touret N, Furuya W, Forbes J, Gros P, Grinstein S (2003) Dynamic traffic through the recycling compartment couples the metal transporter Nramp2 (DMT1) with the transferrin receptor. J Biol Chem 278(28):25548–25557. https://doi.org/10.1074/jbc.M212374200
Lam-Yuk-Tseung S, Touret N, Grinstein S, Gros P (2005) Carboxyl-terminus determinants of the iron transporter DMT1/SLC11A2 isoform II (-IRE/1B) mediate internalization from the plasma membrane into recycling endosomes. Biochemistry 44(36):12149–12159. https://doi.org/10.1021/bi050911r
Kouli A, Horne CB, Williams-Gray CH (2019) Toll-like receptors and their therapeutic potential in Parkinson’s disease and α-synucleinopathies. Brain Behav Immun 81:41–51. https://doi.org/10.1016/j.bbi.2019.06.042
La Vitola P, Balducci C, Cerovic M, Santamaria G, Brandi E, Grandi F, Caldinelli L, Colombo L, Morgese MG, Trabace L, Pollegioni L, Albani D, Forloni G (2018) Alpha-synuclein oligomers impair memory through glial cell activation and via Toll-like receptor 2. Brain Behav Immun 69:591–602. https://doi.org/10.1016/j.bbi.2018.02.012
Choi I, Zhang Y, Seegobin SP, Pruvost M, Wang Q, Purtell K, Zhang B, Yue Z (2020) Microglia clear neuron-released α-synuclein via selective autophagy and prevent neurodegeneration. Nat Commun 11(1):1386. https://doi.org/10.1038/s41467-020-15119-w
Tong WH, Maio N, Zhang DL, Palmieri EM, Ollivierre H, Ghosh MC, McVicar DW, Rouault TA (2018) TLR-activated repression of Fe-S cluster biogenesis drives a metabolic shift and alters histone and tubulin acetylation. Blood Adv 2(10):1146–1156. https://doi.org/10.1182/bloodadvances.2018015669
Colucci S, Pagani A, Pettinato M, Artuso I, Nai A, Camaschella C, Silvestri L (2017) The immunophilin FKBP12 inhibits hepcidin expression by binding the BMP type I receptor ALK2 in hepatocytes. Blood 130(19):2111–2120. https://doi.org/10.1182/blood-2017-04-780692
Billesbølle CB, Azumaya CM, Kretsch RC, Powers AS, Gonen S, Schneider S, Arvedson T, Dror RO, Cheng Y, Manglik A (2020) Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature 586(7831):807–811. https://doi.org/10.1038/s41586-020-2668-z
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529. https://doi.org/10.1038/nrm2199
Bohnert KR, McMillan JD, Kumar A (2018) Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease. J Cell Physiol 233(1):67–78. https://doi.org/10.1002/jcp.25852
Tirasophon W, Welihinda AA, Kaufman RJ (1998) A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev 12(12):1812–1824. https://doi.org/10.1101/gad.12.12.1812
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13(2):89–102. https://doi.org/10.1038/nrm3270
Wang M, Kaufman RJ (2014) The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer 14(9):581–597. https://doi.org/10.1038/nrc3800
Wu J, Kaufman RJ (2006) From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ 13(3):374–384. https://doi.org/10.1038/sj.cdd.4401840
Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, Pietrangelo A (2009) ER stress controls iron metabolism through induction of hepcidin. Science 325(5942):877–880. https://doi.org/10.1126/science.1176639
Kikuchi D, Tanimoto K, Nakayama K (2016) CREB is activated by ER stress and modulates the unfolded protein response by regulating the expression of IRE1α and PERK. Biochem Biophys Res Commun 469(2):243–250. https://doi.org/10.1016/j.bbrc.2015.11.113
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 31871049, 31771124, 31900745), Excellent Innovative Team of Shandong Province and Taishan Scholars Construction Project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mi, X., Li, Q., Wen, X. et al. Extracellular α-Synuclein Modulates Iron Metabolism Related Proteins via Endoplasmic Reticulum Stress in MES23.5 Dopaminergic Cells. Neurochem Res 46, 1502–1513 (2021). https://doi.org/10.1007/s11064-021-03292-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03292-3